Dihydrokawain synthesis
- Product Name:Dihydrokawain
- CAS Number:587-63-3
- Molecular formula:C14H16O3
- Molecular Weight:232.27
A Total Synthesis of (+)- and (–)-Dihydrokavain with a Sonochemical Blaise Reaction as the Key Step
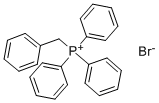
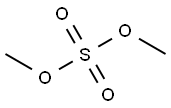
1449-46-3
312 suppliers
$5.00/1g

587-63-3
133 suppliers
$119.00/5mg
Yield:-
Steps:
Multi-step reaction with 8 steps
1.1: nBuLi / hexane; tetrahydrofuran / 0.33 h / 0 °C
1.2: 80 percent / hexane; tetrahydrofuran / 2 h / 20 °C
2.1: 98 percent / H2 / Pd/C / methanol / 8 h / 20 °C
3.1: 97 percent / aq. HCl / methanol / 1 h / 20 °C
4.1: 75 percent / pyridine / 12 h / -17 °C
5.1: 89 percent / ethanol; H2O / 12 h / 20 °C
6.1: Zn / tetrahydrofuran / 0.17 h / 50 °C / ultrasonic irradiation
6.2: 69 percent / tetrahydrofuran / 5.5 h / 50 °C / ultrasonic irradiation
7.1: 84 percent / p-toluenesulfonic acid / CH2Cl2 / 2 h / 20 °C
8.1: 86 percent / K2CO3 / acetone / 20 h / 20 °C
References:
Wang, Fang-Dao;Yue, Jian-Min [European Journal of Organic Chemistry,2005,# 12,p. 2575 - 2579]
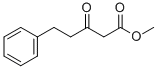
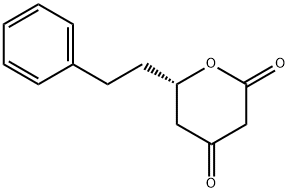
30414-58-5
4 suppliers
inquiry

587-63-3
133 suppliers
$119.00/5mg
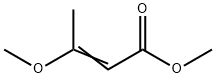
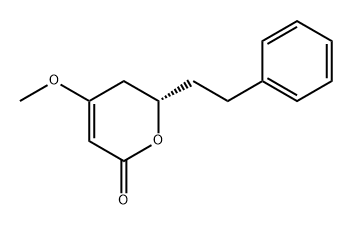
35217-21-1
5 suppliers
inquiry

587-63-3
133 suppliers
$119.00/5mg