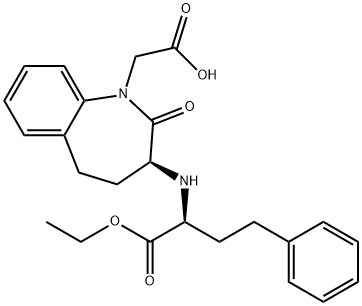
Benazepril synthesis
- Product Name:Benazepril
- CAS Number:86541-75-5
- Molecular formula:C24H28N2O5
- Molecular Weight:424.49

86541-74-4
380 suppliers
$5.00/50mg

86541-75-5
159 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium carbonate in Isopropyl acetate;water at 20; pH=4 - 5;Industry scale;
Steps:
2; 5
Example 2; Preparation of Benazepril To the crude benazepril hydrochloride, water (about 300 gal) and isopropyl acetate (4600 Ib) were added, forming a biphasic solution. The temperature was kept below 2O0C during these additions. Aqueous potassium carbonate (47% K2CO3, approximately 500 Ib) was then added until the pH was between 4.2 and 4.8, measured internally, producing a solution of benazepril in isopropyl acetate and an aqueous layer. The aqueous layer was then removed, and was back-extracted with a portion of isopropyl acetate, which was added to the benazepril solution. This solution was washed once with water, and then the solvent was removed by distillation. The distillation was started at atmospheric pressure, and as soon as the temperature of the distillation pot reached 88°C, heating was stopped. The pot was then placed under partial vacuum, and distillation was continued under 23 in. Hg of relative vacuum until substantially all of the isopropyl acetate had been removed, without permitting the distillation pot temperature to exceed 600C. Example 5; Crude benazepril hydrochloride was prepared by the method described in Example 1 , except that the crude product was not washed with isopropyl acetate before proceeding with the process described in Example 2.To the crude benazepril hydrochloride, water and isopropyl acetate were added, forming a biphasic solution. The temperature was kept below 200C during these additions. Aqueous potassium carbonate (47% K2CO3, approximately 500 Ib) was then added until the pH was between about 4 and 5, producing a solution of benazepril in isopropyl acetate. The aqueous layer was then removed, and was back- extracted with a portion of isopropyl acetate, which was added to the benazepril solution. This solution was washed once with water, and then the solvent was removed by distillation. After the isopropyl acetate-water azeotrope had been distilled off, removal of isopropyl acetate was continued at 88-900C for 2-4 hours. The pot was then placed under about 20 in. Hg of relative vacuum and distillation was continued until substantially all of the isopropyl acetate had been removed. Using the procedure described in Example 3, benazepril hydrochloride of Form B was isolated.
References:
WO2006/84761,2006,A1 Location in patent:Page/Page column 22; 23
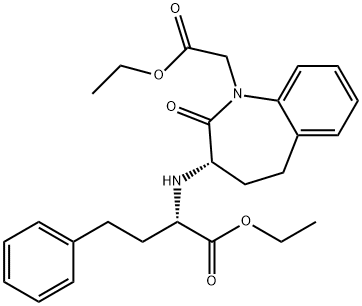
103129-58-4
56 suppliers
$149.90/10mg

86541-75-5
159 suppliers
inquiry
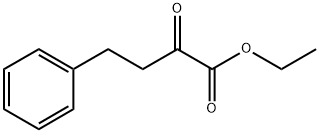
64920-29-2
255 suppliers
$10.00/1g

86541-75-5
159 suppliers
inquiry