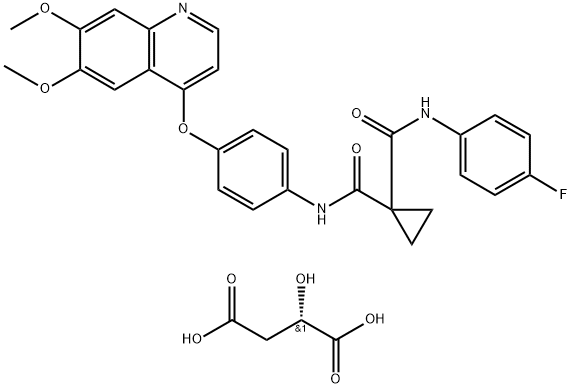
Cabozantinib Malate synthesis
- Product Name:Cabozantinib Malate
- CAS Number:1140909-48-3
- Molecular formula:C32H30FN3O10
- Molecular Weight:635.6

The preparation began with 6,7-dimethoxy-quinoline-4-ol (46) which upon treatment with POCl3 provided chloride 47 in 70% yield. Exposure of 47 to 4-aminophenol under basic conditions using t- BuONa furnished diaryl ether 48 in 72% yield. This aniline was then coupled with amidoacid chloride 51 (which arose from the activation of commercial diacid 49 to the corresponding monochloride and coupling with p-fluoroaniline and subsequent exposure to oxalyl chloride to furnish the transient acid chloride) to construct cabozantinib as the free base 52 in 95% yield. Salt formation of cabozantinib 52 was carried out with (S)-malic acid, which ultimately delivered the final product of cabozantinib (S)- malate (VIII) in 75% yield.
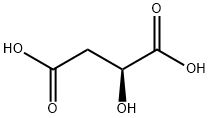
97-67-6
620 suppliers
$5.00/25g
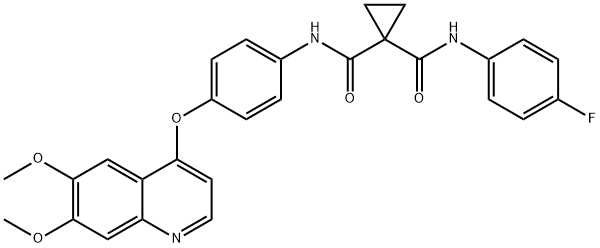
849217-68-1
414 suppliers
$7.00/10mg

1140909-48-3
325 suppliers
inquiry
Yield:1140909-48-3 92.26%
Reaction Conditions:
in tetrahydrofuran at 50 - 70;Large scale;Solvent;
Steps:
3; 6 Example 6: Synthesis of Cabozantinib Malate
Add 800.0g of cabozantinib to 12.0L of tetrahydrofuran and raise the temperature to 5070,Add 264.7g of L-malic acid solid directly, continue to stir for 23h, cool to 1020, stir for 12h, filter, rinse with 1.6L tetrahydrofuran,The filter cake was vacuum dried at 50±5°C to a constant weight to obtain 935.4 g of cabozantinib malate.The HPLC purity is 99.91%, the yield is 92.26%, and the maximum single impurity is 0.02% (see Figure 6 for the HPLC chart).
References:
CN112979544,2021,A Location in patent:Paragraph 0042-0044; 0049-0050
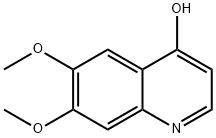
13425-93-9
268 suppliers
$6.00/1g

1140909-48-3
325 suppliers
inquiry
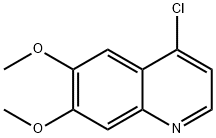
35654-56-9
353 suppliers
$13.00/1g

1140909-48-3
325 suppliers
inquiry
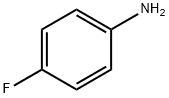
371-40-4
409 suppliers
$5.00/5G

1140909-48-3
325 suppliers
inquiry
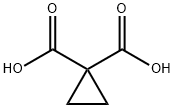
598-10-7
356 suppliers
$7.00/1g

1140909-48-3
325 suppliers
inquiry