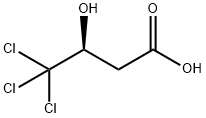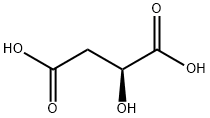
L-Malic acid synthesis
- Product Name:L-Malic acid
- CAS Number:97-67-6
- Molecular formula:C4H6O5
- Molecular Weight:134.09
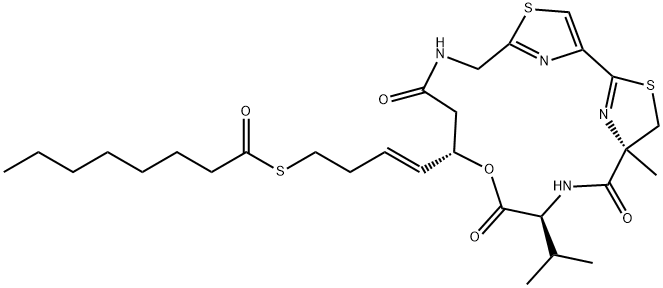
1009815-87-5
5 suppliers
inquiry
640-68-6
461 suppliers
$8.00/10g
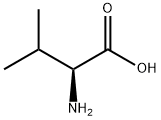
72-18-4
858 suppliers
$5.00/25g

97-67-6
638 suppliers
$5.00/25g
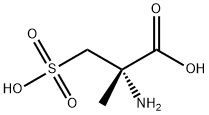
1127229-10-0
0 suppliers
inquiry
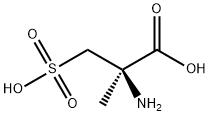
1127229-09-7
0 suppliers
inquiry
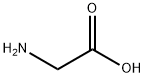
56-40-6
1278 suppliers
$5.00/25g
Yield:-
Reaction Conditions:
Stage #1: largazolewith ozone in dichloromethane at 25; for 0.5 h;
Stage #2: with formic acid;dihydrogen peroxide at 70; for 0.333333 h;
Stage #3: with hydrogenchloride;water at 110; for 24 h;
Steps:
3
Example 3: Determination of Absolute Configuration. A sample of compound 21 (~100 μg) was dissolved in 4 mL Of CH2Cl2 and subjected to ozonolysis at room temperature for 30 min. The solvent was evaporated and the residue was treated with 0.6 mL Of H2O2-HCO2H (1 :2) at 70 0C for 20 min. The solvent was evaporated and the resulting oxidation product was hydrolyzed with 0.5 mL of 6 N HCl at 1 10 0C for 24 h. The hydrolyzed product was dried and analyzed by chiral HPLC (column, Phenomenex Chirex phase 3126 N.S-dioctyl-φ)- pencillamine, 4.60 x 250 mm, 5 μm; solvent 1, 2 mM CuSO4 in 95:5 H2O/MeCν, pH 4.50; solvent 2, 0.5 mM Cu(OAc)2/0.1 M NH4OAc in 85:15 H2O/MeCN, pH 4.6; flow rate 1.0 mL/min; detection at 254 nm). The absolute configuration of the amino
References:
WO2009/105284,2009,A1 Location in patent:Page/Page column 24-25
328-42-7
270 suppliers
$8.00/250mg

97-67-6
638 suppliers
$5.00/25g
56-84-8
912 suppliers
$5.00/25g

97-67-6
638 suppliers
$5.00/25g
110-17-8
1081 suppliers
$9.00/5g

97-67-6
638 suppliers
$5.00/25g