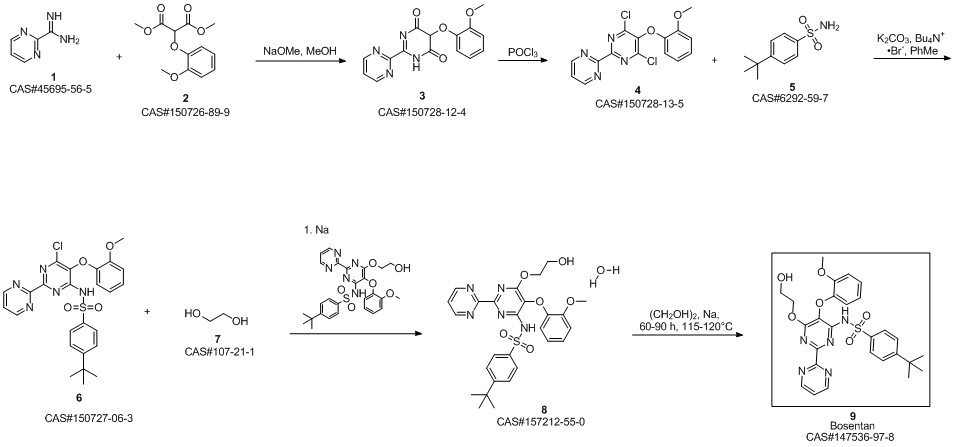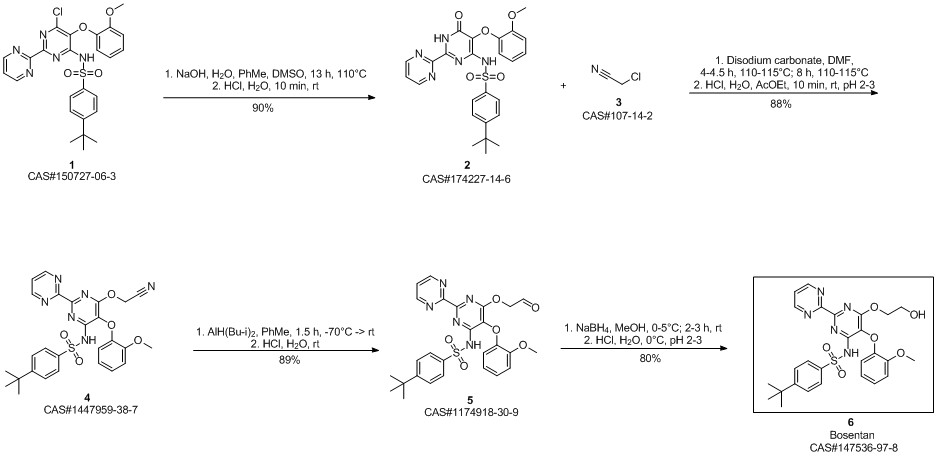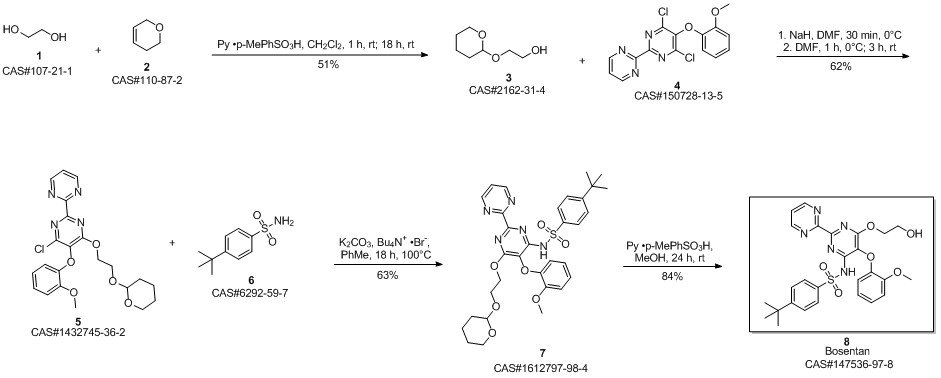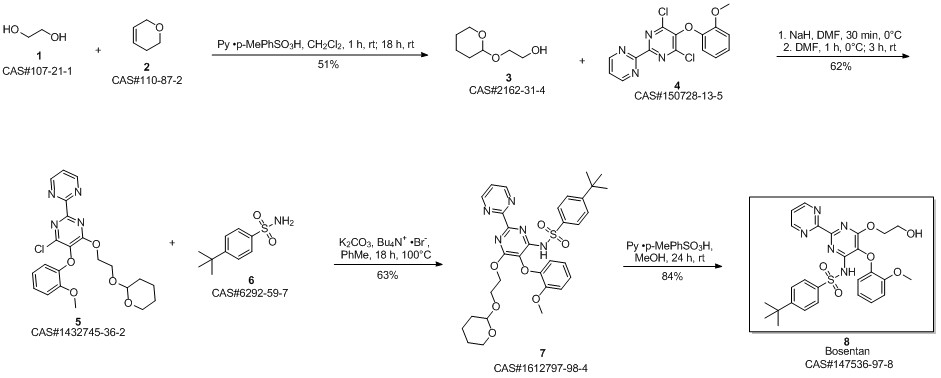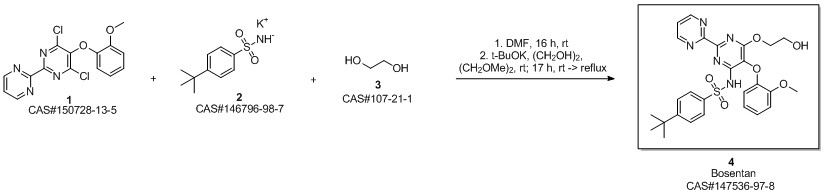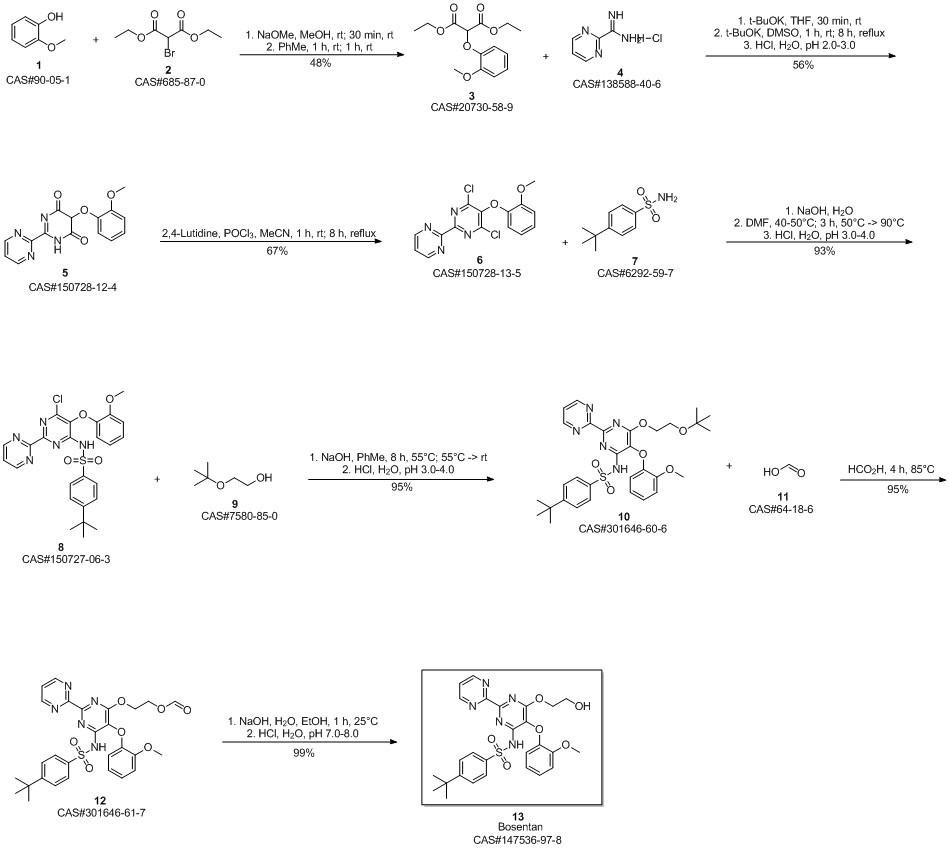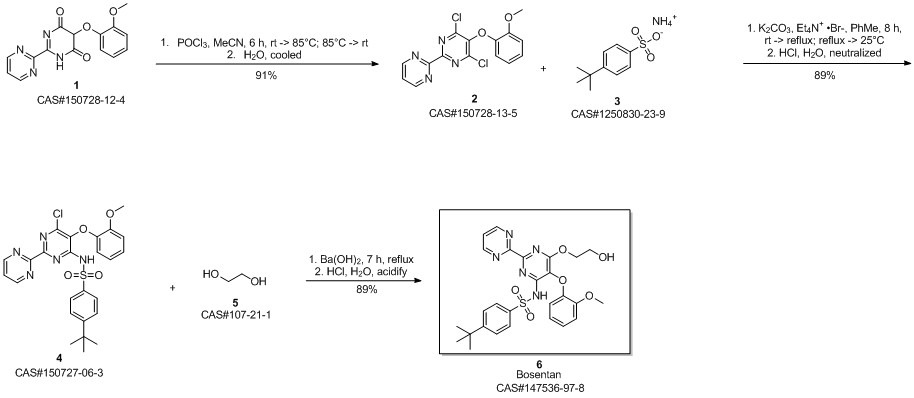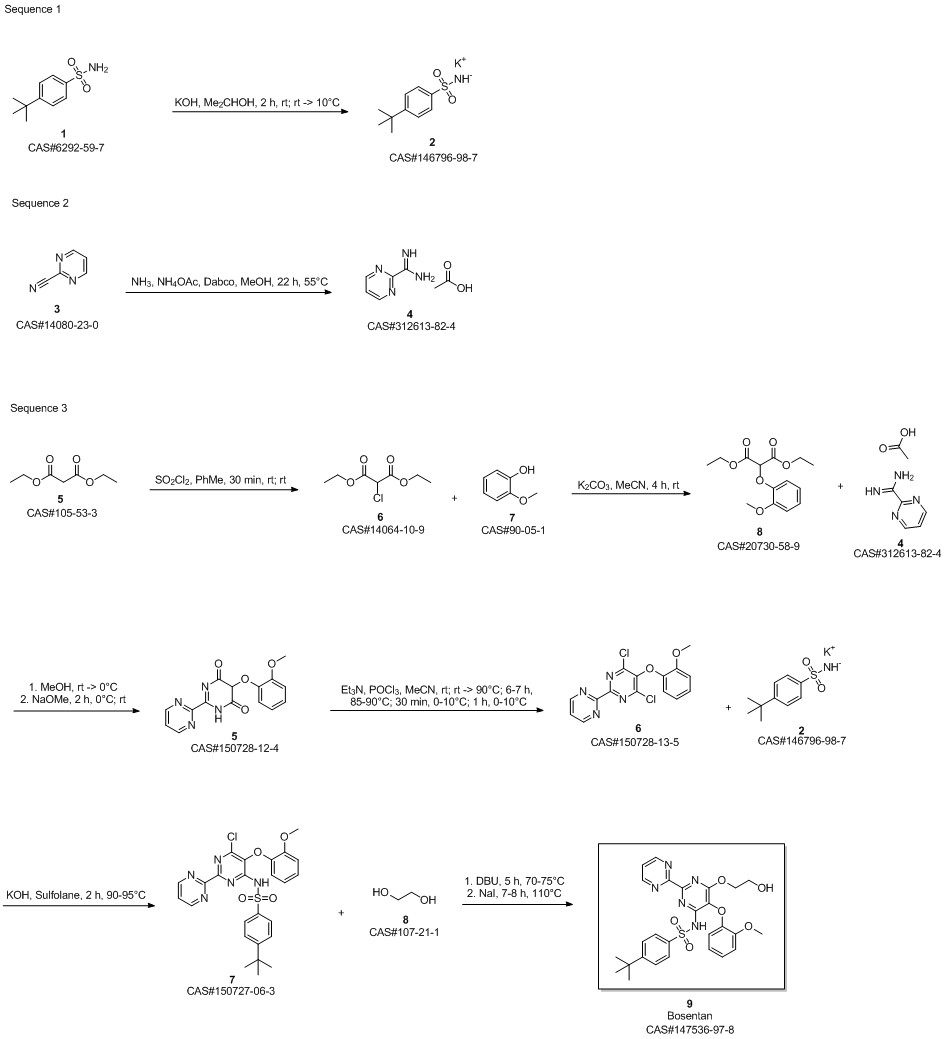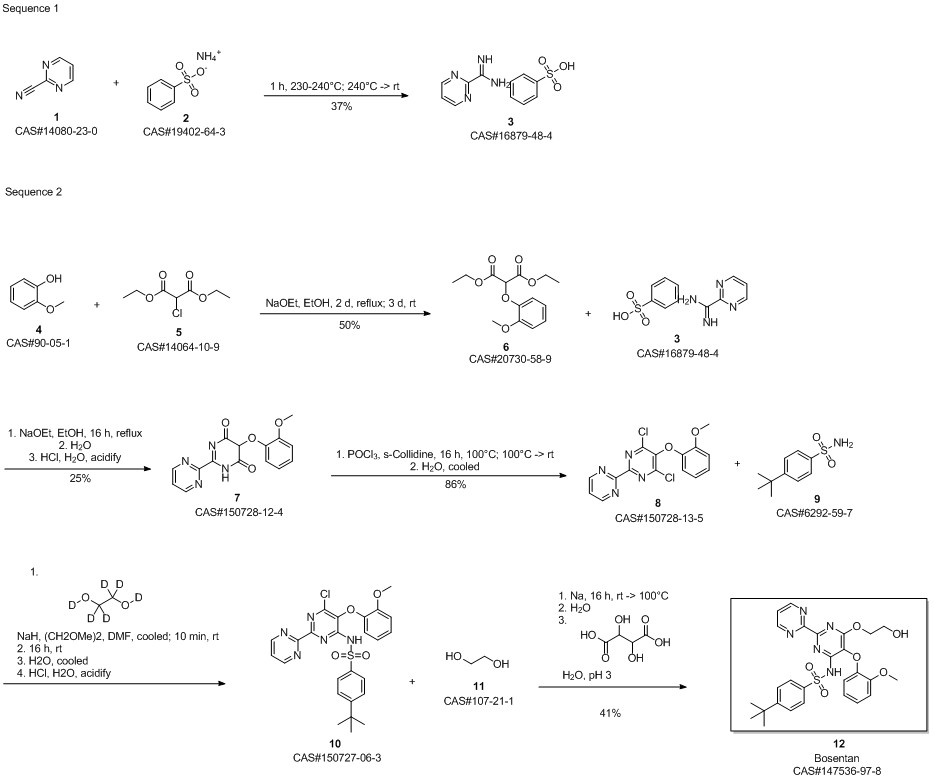
Bosentan synthesis
- Product Name:Bosentan
- CAS Number:147536-97-8
- Molecular formula:C27H29N5O6S
- Molecular Weight:551.61
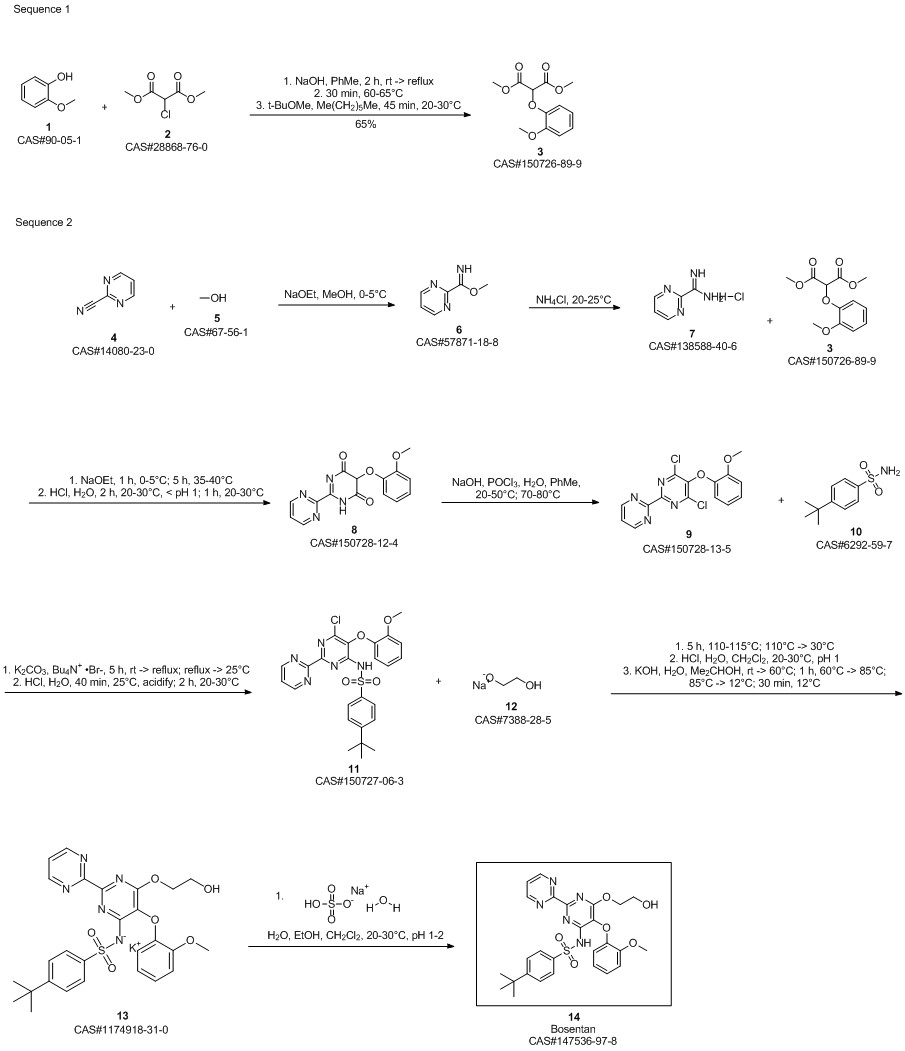
Raju, K. Rajasekhara; Reddy, B. Shankar; Somannavar, Y. S.; Sinha, B. K.; Babu, P. N. Kishore; Raju, K. Mohana. Improved Large-Scale Synthesis of Bosentan Monohydrate. Organic Preparations and Procedures International. Chemical Research and Development. Aurobindo Pharma Ltd. Telangana, India. Volume 48. Issue 6. Pages 481-491. 2016

107-21-1
1339 suppliers
$10.00/25g
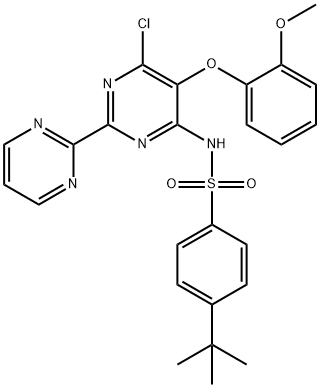
150727-06-3
160 suppliers
$17.00/100mg

147536-97-8
244 suppliers
$29.00/5mg
Yield:147536-97-8 95.7%
Reaction Conditions:
Stage #1: ethylene glycol;4-tert-butyl-N-(6-chloro-5-(2-methoxyphenoxy)2,2'-bipyrimidin-4-yl)benzene sulfonamidewith sodium hydroxide in tetrahydrofuran at 60 - 65; for 12 h;
Stage #2: with tartaric acid in tetrahydrofuran;water at 5 - 10; for 1 h;
Steps:
8
Example 8: Preparation of crude Bosentan from compound of formula Via.The compound of formula Via (130.0g, 1.0eq), ethylene glycol (1470.0ml,107.0eq) and sodium hydroxide (88.9g, 9.0eq) were heated in tetrahydrofuran (1560.0ml,12.0vol) at 60- 65°C for 12hours. After completion of reaction, the reaction mixture was added to a mixture of water (4450.0ml,35.0vol) and DL-Tartaric acid (185.0g, 5.0eq) at 5-10°C. Maintained at this temperature for 1 hour. Filtered the solid and washed with water (1300.0ml). Dried the solid, Bosentan, under vacuum at 50-55°C for 12 hours to get the title compound (Yield: 134.5g (95.7%, HPLC purity 98.41 %).
References:
WO2012/56468,2012,A1 Location in patent:Page/Page column 16
![Benzenesulfonamide, 4-(1,1-dimethylethyl)-N-[6-(2-hydroxyethoxy)-2-iodo-5-(2-methoxyphenoxy)-4-pyrimidinyl]-](/CAS/20210111/GIF/1206769-86-9.gif)
1206769-86-9
1 suppliers
inquiry
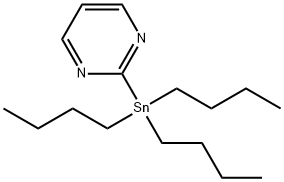
153435-63-3
108 suppliers
$35.00/250mg

147536-97-8
244 suppliers
$29.00/5mg
![Benzenesulfonamide, N-[2-bromo-6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-4-pyrimidinyl]-4-(1,1-dimethylethyl)-](/CAS/20210111/GIF/1206769-81-4.gif)
1206769-81-4
1 suppliers
inquiry

153435-63-3
108 suppliers
$35.00/250mg

147536-97-8
244 suppliers
$29.00/5mg
![Potassium ((4-(tert-butyl)phenyl)sulfonyl)(6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-[2,2''-bipyrimidin]-4-yl)amide](/CAS/20211123/GIF/1174918-31-0.gif)
1174918-31-0
1 suppliers
inquiry

147536-97-8
244 suppliers
$29.00/5mg
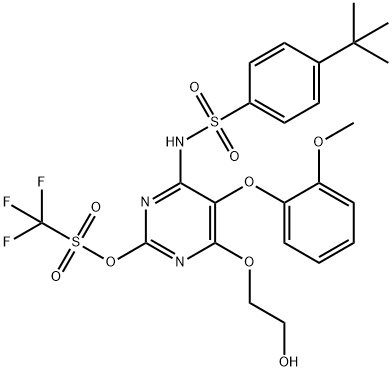
1334686-05-3
1 suppliers
inquiry

153435-63-3
108 suppliers
$35.00/250mg

147536-97-8
244 suppliers
$29.00/5mg