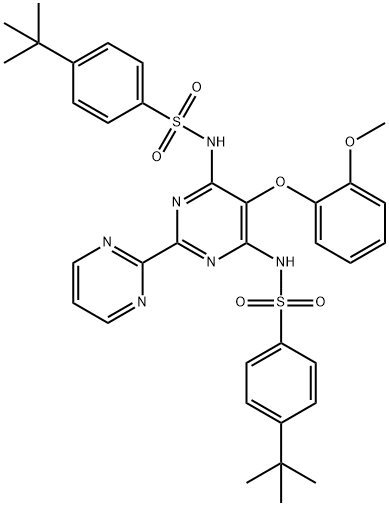
Bosentan Impurity 1 synthesis
- Product Name:Bosentan Impurity 1
- CAS Number:1218951-81-5
- Molecular formula:C35H38N6O6S2
- Molecular Weight:702.84

6292-59-7
316 suppliers
$10.00/5g
150727-06-3
166 suppliers
$17.00/100mg

1218951-81-5
14 suppliers
inquiry
Yield:-
Reaction Conditions:
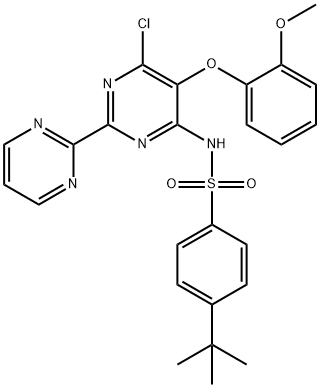
Stage #1: 4-tert-butylbenzenesulphonamide;4-tert-butyl-N-(6-chloro-5-(2-methoxyphenoxy)2,2'-bipyrimidin-4-yl)benzene sulfonamidewith potassium carbonate in N,N-dimethyl-formamide at 145 - 150; for 40 h;
Stage #2: with hydrogenchloride in water; pH=3; for 0.25 h;
Steps:
50; 4
In 250 mL 3 neck round bottom flask, 6.5g N-(6-Chloro-5-(2- methoxyphenoxy)[2,2'-bipyrimidinyl]-4-/-butyl benzene sulfonamide, 3.9 g 4-t-Butyl benzene sulphonamide and 65 mL DMF was taken at room temperature. The reaction mixture was heated at 145-150 0C. 1.87 g anhydrous K2CO3 was added to the reaction mixture and heated at 145-150 0C for 16 hours. Further 2.69 g 4-t-butyl benzene sulphonamide and 1.19 g anhydrous K2CO3 was added and further reaction mixture was heated at 145-150 0C for 24 hrs. The reaction mixture was cooled to room temperature and dumped into water. Yellow solid was precipitated out which was stirred for 15 min. The reaction mixture was acidified with 50 % HCl till pH 3 and stirred for 15 min. Sticky solid was formed which was isolated with ethyl acetate. Free flowing yellow material was obtained. Solid was filtered and washed with water and then washed with ethyl acetate. Yield:- 2.5 g, HPLC: 95.6 %, unwanted material and only 1 % N,N'-(5- (2-methoxyphenoxy)-2,2'-bipyrimidine-4,6-diyl)bis(4-tert-butylbenzenesulfonamide).Filtrate was combined and ethyl acetate layer was separated out. Ethyl acetate layer was washed with water, followed by brine, dried over anhydrous sulfate and solvent was removed under vacuum to give 7.4 g, N,N'-(5-(2-methoxyphenoxy)-2,2'- bipyrimidine-4,6-diyl)bis(4-tert-butylbenzenesulfonamide), HPLC Purity= 11.14 %.(6g) of N,N'-(5-(2-methoxyphenoxy)-2,2'-bipyrimidine-4,6-diyl)-bis(4-tert- butylbenzenesulfonamide) was taken in 50 mL conical flask and 40 mL isopropyl alcohol was added and stirred for 5 min., when a suspension was formed. The reaction mixture was heated at 80 0C for 5 min. to obtain clear solution. The solution was allowed to stand at room temperature for 2 hrs and yellow solid was precipitated. Solid product was filtered and washed with 10 ml isopropyl alcohol and dried. Solid was obtained (700mg), which contains 3.65% N,N'-(5-(2-methoxyphenoxy)-2,2'- bipyrimidine-4,6-diyl)bis(4-tert-butylbenzenesulfonamide). Filtrate was concentrated to give 4.8 g N,N'-(5-(2-methoxyphenoxy)-2,2'-bipyrimidine-4,6-diyl)bis(4-tert- butylbenzenesulfonamide). This was further purified with methanol to give 950 mg N,N'-(5-(2-methoxyphenoxy)-2,2'-bipyrimidine-4,6-diyl)bis(4-tert- butylbenzenesulfonamide). (LC / MS result):Bis sulphonamide impurity = RT= 24.304, % Area-30.61% I O15
References:
WO2010/32261,2010,A1 Location in patent:Page/Page column 11-12; 22-23