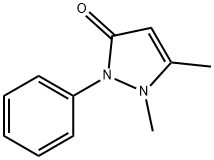
Antipyrine synthesis
- Product Name:Antipyrine
- CAS Number:60-80-0
- Molecular formula:C11H12N2O
- Molecular Weight:188.23
Yield:60-80-0 58%
Reaction Conditions:
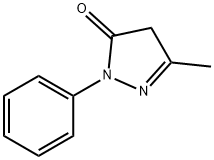
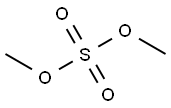
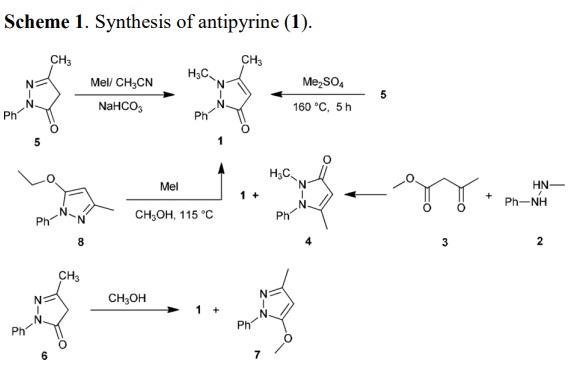
Stage #1:3-methyl-1-phenylpyrazolin-5-(4H)-one;methyl iodide in acetonitrile at 120;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate
Steps:
16
In a stainless steel pressure bomb the pyrazalone (1.0 equiv.) in acetonitrile was set stirring with iodomethane (5.0 equiv.) in a 12O0C oil bath overnight. The crude product was added to saturated sodium bicarbonate and then extracted four times into ethyl acetate. After concentration, the crude product was chromatographed in a mixture of dichloromethane and methanol. NMR was used to determine the purity of the isolated compounds.; I3 5-Dimethyl-2-phenyl-l, 2-dihydropyrazol-3-one was obtained from 5-methyl-2-phenyl-l, 2-dihydropyrazol-3-one (3.52 g, 20 mmol) and iodomethane (3.38 mL, 60 mmol) in acetonitrile (20 mL) as an off-white solid (2.2 g, 58%). 1H NMR (300 MHz, CDCl3): δ(ppm) 7.26 - 7.49 (m 5H), 5.41 (s, IH), 3.07 (s, 3H), 2.25 (s, 3H).
References:
ASTRAZENECA AB;NPS PHARMACEUTICALS, INC. WO2006/71730, 2006, A1 Location in patent:Page/Page column 50
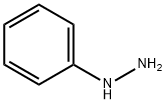
100-63-0
384 suppliers
$10.00/1g

60-80-0
489 suppliers
$10.00/5g