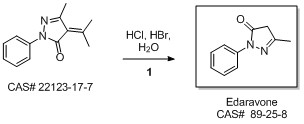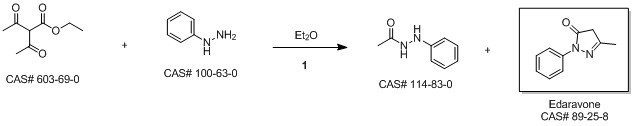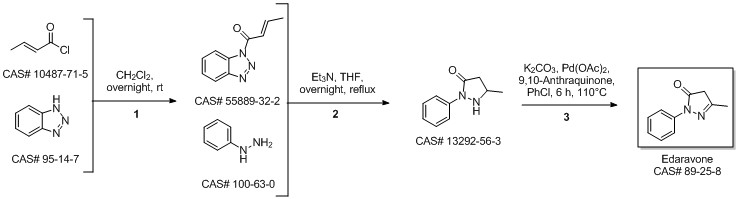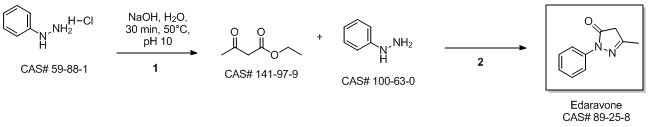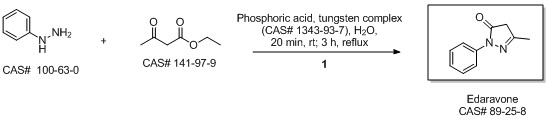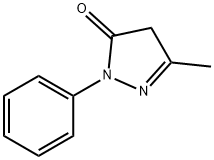
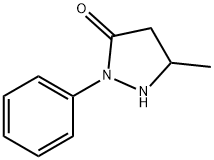
5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one synthesis
- Product Name:5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one
- CAS Number:89-25-8
- Molecular formula:C10H10N2O
- Molecular Weight:174.2
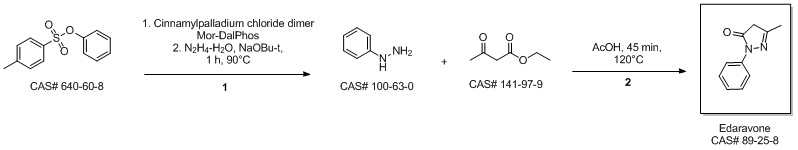
MacLean, Mark A.; Diez-Cecilia, Elena; Lavery, Christopher B.; Reed, Mark A.; Wang, Yanfei; Weaver, Donald F.; Stradiotto, Mark. Diversification of edaravone via palladium-catalyzed hydrazine cross-coupling: Applications against protein misfolding and oligomerization of beta-amyloid. Bioorganic & Medicinal Chemistry Letters. Department of Chemistry. Dalhousie University. Halifax, Can. B3H 4R2. Volume 26. Issue 1. Pages 100-104. 2016.
Yield:89-25-8 100%
Reaction Conditions:
at 0 - 90; for 1.5 h;
Steps:
2.2 Synthesis of 1-phenyl-3-methyl-5-pyrazolone
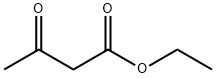
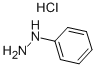
Ethyl acetoacetate (28.1 mL, 28.63 g, 0.22 mol) wasplaced in a 100 mL one necked flask equipped with amagnetic stirrer and immersed in an ice-water bath(0 C). Then, phenyl hydrazine (19.66 mL, 21.63 g,0.20 mol) was dropwise added (1 mL/min). After theaddition of phenyl hydrazine, the flask cap has beentightly closed and the reaction was continued for 1 h at80 C, and for 30 min at 90 C. Finally, water, ethanoland excess ethyl acetoacetate were vacuum strippedand the formed solids were washed with diethyl ether(20 mL) giving pale yellow solids (34.8 g, R=*100%). M.p. 126-128 C. 1H NMR (400 MHz,CDCl3, d ppm): 2.18 (s, 3H), 3.42 (s, 2H), 7.17 (t, 1H,J=6.7 Hz), 7.38 (t, 2H, J=6.7 Hz), 7.84 (d, 2H, J=7.5Hz).13C NMR (100 MHz, CDCl3, dppm): 16.6, 42.6,118.4, 124.6, 128.4, 137.6, 156.1, 170.2.
References:
Fakhraian, Hossein;Nafari, Yaser [Journal of Chemical Sciences,2021,vol. 133,# 2,art. no. 40]
201230-82-2
1 suppliers
inquiry
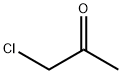
78-95-5
0 suppliers
$21.50/5G
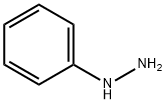
100-63-0
384 suppliers
$10.00/1g

89-25-8
824 suppliers
$6.00/25g
13292-56-3
18 suppliers
inquiry

89-25-8
824 suppliers
$6.00/25g