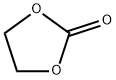
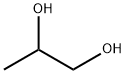
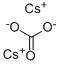
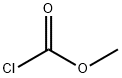
Yield:616-38-6 99.88%
Reaction Conditions:
potassium hydroxide in water at 98; under 838.584 Torr; for 500 - 6000 h;Product distribution / selectivity;Heating / reflux;
Steps:
1; 2; 3; 4
Example 1: A continuous multi-stage distillation column as shown in FIG. 1 having L = 3300 cm, D = 300 cm, L / D = 11, n = 60, D / d1 = 7.5, and D / d2 = 12 was used. The trays in the distillation column were sieve trays, each having the cross-sectional area per hole in the sieve portion thereof of approximately 1.3 cm2 and a number of holes of approximately 180 to 32 / m2. The aperture ratio of each of the trays was in a range of from 2.1 to 4.2%. 3.27 Ton / hr of ethylene carbonate in a liquid form was continuously introduced into the distillation column from an inlet (3-a) provided at the 55th stage from the bottom. 3.238 Ton / hr of methanol in a gaseous form (containing 8.96 % by weight of dimethyl carbonate) and 7.489 ton / hr of methanol in a liquid form (containing 6.66 % by weight of dimethyl carbonate) were respectively continuously introduced into the distillation column from inlets (3-b and 3-c) provided at the 31st stage from the bottom. The molar ratio of the starting materials introduced into the distillation column was methanol / ethylene carbonate = 8.36. The catalyst used was obtained by adding 4.8 ton of ethylene glycol to 2.5 ton of KOH (48 % by weight aqueous solution), heating to approximately 130 C, gradually reducing the pressure, and carrying out heat treatment for approximately 3 hours at approximately 1300 Pa, so as to produce a homogeneous solution. This catalyst solution was continuously introduced into the distillation column from an inlet (3-e) provided at the 54th stage from the bottom (K concentration: 0.1 % by weight, based on ethylene carbonate fed in). Reactive distillation was carried out continuously under conditions of a column bottom temperature of 98 C, a column top pressure of approximately 1.118x105 Pa, and a reflux ratio of 0.42. It was possible to attain stable steady state operation after 24 hours. A low boiling point reaction mixture withdrawn from the top 1 of the column in a gaseous form was cooled using a heat exchanger and thus turned into a liquid. The liquid low boiling point reaction mixture, which was continuously withdrawn from the distillation column at 10.678 ton / hr, contained 4.129 ton / hr of dimethyl carbonate, and 6.549 ton / hr of methanol. A liquid continuously withdrawn from the bottom 2 of the column at 3.382 ton / hr contained 2.356 ton / hr of ethylene glycol, 1.014 ton / hr of methanol, and 4 kg / hr of unreacted ethylene carbonate. Excluding the dimethyl carbonate contained in the starting material, the actual produced amount of dimethyl carbonate was 3.340 ton / hr, and excluding the ethylene glycol contained in the catalyst solution, the actual produced amount of ethylene glycol was 2.301 ton / hr. The ethylene carbonate conversion was 99.88%, the dimethyl carbonate selectivity was not less than 99.99%, and the ethylene glycol selectivity was not less than 99.99%. Prolonged continuous operation was carried out under these conditions. After 500 hours, 2000 hours, 4000 hours, 5000 hours, and 6000 hours, the actual produced amounts per hour were 3.340 ton, 3.340 ton, 3.340 ton, 3.340 ton, and 3.340 ton respectively for dimethyl carbonate, and 2.301 ton, 2.301 ton, 2.301 ton, 2.301 ton, and 2.301 ton respectively for ethylene glycol, the ethylene carbonate conversions were respectively 99.90%, 99.89%, 99.89%, 99.88%, and 99.88%, the dimethyl carbonate selectivities were respectively not less than 99.99%, not less than 99.99%, not less than 99.99%, not less than 99.99%, and not less than 99.99%, and the ethylene glycol selectivities were respectively not less than 99.99%, not less than 99.99%, not less than 99.99%, not less than 99.99%, and not less than 99.99%.Example 2: Reactive distillation was carried out under the following conditions using the same continuous multi-stage distillation column as in Example 1. 2.61 Ton / hr of ethylene carbonate in a liquid form was continuously introduced into the distillation column from the inlet (3-a) provided at the 55th stage from the bottom. 4.233 Ton / hr of methanol in a gaseous form (containing 2.41 % by weight of dimethyl carbonate) and 4.227 ton / hr of methanol in a liquid form (containing 1.46 % by weight of dimethyl carbonate) were respectively continuously introduced into the distillation column from the inlets (3-b and 3-c) provided at the 31st stage from the bottom. The molar ratio of the starting materials introduced into the distillation column was methanol / ethylene carbonate = 8.73. The catalyst was made to be the same as in Example 1, and was continuously fed into the distillation column. Reactive distillation was carried out continuously under conditions of a column bottom temperature of 93 C, a column top pressure of approximately 1.046*105 Pa, and a reflux ratio of 0.48. It was possible to attain stable steady state operation after 24 hours. A low boiling point reaction mixture withdrawn from the top 1 of the column in a gaseous form was cooled using a heat exchanger and thus turned into a liquid. The liquid low boiling point reaction mixture, which was continuously withdrawn from the distillation column at 8.17 ton / hr, contained 2.84 ton / hr of dimethyl carbonate, and 5.33 ton / hr of methanol. A liquid continuously withdrawn from the bottom 2 of the column at 2.937 ton / hr contained 1.865 ton / hr of ethylene glycol, 1.062 ton / hr of methanol, and 0.2 kg / hr of unreacted ethylene carbonate. Excluding the dimethyl carbonate contained in the starting material, the actual produced amount of dimethyl carbonate was 2.669 ton / hr, and excluding the ethylene glycol contained in the catalyst solution, the actual produced amount of ethylene glycol was 1.839 ton / hr. The ethylene carbonate conversion was 99.99%, the dimethyl carbonate selectivity was not less than 99.99%, and the ethylene glycol selectivity was not less than 99.99%. Prolonged continuous operation was carried out under these conditions. After 1000 hours, 2000 hours, 3000 hours, and 5000 hours, the actual produced amounts per hour were 2.669 ton, 2.669 ton, 2.669 ton, and 2.669 ton respectively for dimethyl carbonate, and 1.839 ton, 1.839 ton, 1.839 ton, and 1.839 ton respectively for ethylene glycol, the ethylene carbonate conversions were respectively 99.99%, 99.99%, 99.99%, and 99.99%, the dimethyl carbonate selectivities were respectively not less than 99.99%, not less than 99.99%, not less than 99.99%, and not less than 99.99%, and the ethylene glycol selectivities were respectively not less than 99.99%, not less than 99.99%, not less than 99.99%, and not less than 99.99%.Example 3: The continuous multi-stage distillation column as shown in FIG. 1 having L = 3300 cm, D = 300 cm, L / D = 11, n = 60, D / d1 = 7.5, and D / d2 = 12 was used. The trays in the distillation column were sieve trays, each having the cross-sectional area per hole in the sieve portion thereof of approximately 1.3 cm2 and a number of holes of approximately 220 to 340 / m2. The aperture ratio of each of the trays was in a range of from 2.5 to 4.5%. 3.773 Ton / hr of ethylene carbonate in a liquid form was continuously introduced into the distillation column from the inlet (3-a) provided at the 55th stage from the bottom. 3.736 Ton / hr of methanol in a gaseous form (containing 8.97 % by weight of dimethyl carbonate) and 8.641 ton / hr of methanol in a liquid form (containing 6.65 % by weight of dimethyl carbonate) were respectively continuously introduced into the distillation column from the inlets (3-b and 3-c) provided at the 31st stage from the bottom. The molar ratio of the starting materials introduced into the distillation column was methanol / ethylene carbonate = 8.73. The catalyst was made to be the same as in Example 1, and was continuously fed into the distillation column. Reactive distillation was carried out continuously under conditions of a column bottom temperature of 98 C, a column top pressure of approximately 1.118*105 Pa, and a reflux ratio of 0.42. It was possible to attain stable steady state operation after 24 hours. A low boiling point reaction mixture withdrawn from the top of the column in a gaseous form was cooled using a heat exchanger and thus turned into a liquid. The liquid low boiling point reaction mixture, which was continuously withdrawn from the distillation column at 12.32 ton / hr, contained 4.764 ton / hr of dimethyl carbonate, and 7.556 ton / hr of methanol. A liquid continuously withdrawn from the bottom of the column at 3.902 ton / hr contained 2.718 ton / hr of ethylene glycol, 1.17 ton / hr of methanol, and 4.6 kg / hr of unreacted ethylene carbonate. Excluding the dimethyl carbonate contained in the starting material, the actual produced amount of dimethyl carbonate was 3.854 ton / hr, and excluding the ethylene glycol contained in the catalyst solution, the actual produced amount of ethylene glycol was 2.655 ton / hr. The ethylene carbonate conversion was 99.88%, the dimethyl carbonate selectivity was not less than 99.99%, and the ethylene glycol selectivity was not less than 99.99%. Prolonged continuous operation was carried out under these conditions. After 1000 hours, 2000 hours, 3000 hours, and 5000 hours, the actual produced amounts per hour were 3.854 ton, 3.854 ton, 3.854 ton, and 3.854 ton respectively for dimethyl carbonate, and 2.655 ton, 2.655 ton, 2.655 ton, and 2.655 ton respectively for ethylene glycol, the ethylene carbonate conversions were respectively 99.99%, 99.99%, 99.99%, and 99.99%, the dimethyl carbonate selectivities were respectively not less than 99.99%, not less than 99.99%, not less than 99.99%, and not less than 99.99%, and the ethylene glycol selectivities were respectively not less than 99.99%, not less than 99.99%, not less than 99.99%, and not less than 99.99%.Example 4: The continuous multi-stage distillation column as shown in FIG. 1 having L = 3300 cm, D = 300 cm, L / D = 11, n = 60, D/d1 = 7.5, and D / d2 = 12 was used. The trays in the distillation column were sieve trays, each having the cross-sectional area per hole in the sieve portion thereof of approximately 1.3 cm2 and a number of holes of approximately 240 to 360 / m2. The aperture ratio of each of the trays was in a range of from 3.0 to 5.0%. 7.546 Ton / hr of ethylene carbonate in a liquid form was continuously introduced into the distillation column from the inlet (3-a) provided at the 55th stage from the bottom. 7.742 Ton / hr of methanol in a gaseous form (containing 8.95 % by weight of dimethyl carbonate) and 17.282 ton/hr of methanol in a liquid form (containing 6.66 % by weight of dimethyl carbonate) were respectively continuously introduced into the distillation column from the inlets (3-b and 3-c) provided at the 31st stage from the bottom. The molar ratio of the starting materials introduced into the distillation column was methanol / ethylene carbonate = 8.36. The catalyst was made to be the same as in Example 1, and was continuously fed into the distillation column. Reactive distillation was carried out continuously under conditions of a column top temperature of 65 C, a column top pressure of approximately 1.118*105 Pa, and a reflux ratio of 0.42. It was possible to attain stable steady state operation after 24 hours. A low boiling point reaction mixture withdrawn from the top 1 of the column in a gaseous form was cooled using a heat exchanger and thus turned into a liquid. The liquid low boiling point reaction mixture, which was continuously withdrawn from the distillation column at 24.641 ton / hr, contained 9.527 ton / hr of dimethyl carbonate, and 15.114 ton / hr of methanol. A liquid continuously withdrawn from the bottom 2 of the column at 7.804 ton / hr contained 5.436 ton / hr of ethylene glycol, 2.34 ton / hr of methanol, and 23 kg / hr of unreacted ethylene carbonate. Excluding the dimethyl carbonate contained in the starting material, the actual produced amount of dimethyl carbonate was 7.708 ton / hr, and excluding the ethylene glycol contained in the catalyst solution, the actual produced amount of ethylene glycol was 5.31 ton / hr. The ethylene carbonate conversion was 99.7%, the dimethyl carbonate selectivity was not less than 99.99%, and the ethylene glycol selectivity was not less than 99.99%. Prolonged continuous operation was carried out under these conditions. After 1000 hours, the actual produced amount per hour was 7.708 ton for dimethyl carbonate, and 5.31 ton for ethylene glycol, the ethylene carbonate conversion was 99.8%, the dimethyl carbonate selectivity was not less than 99.99%, and the ethylene glycol selectivity was not less than 99.99%.
References:
EP1961721,2008,A1 Location in patent:Page/Page column 15-17