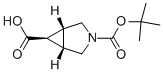
(1R,5S,6R)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.1.0]hexane-6-carboxylic acid synthesis
- Product Name:(1R,5S,6R)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.1.0]hexane-6-carboxylic acid
- CAS Number:927679-54-7
- Molecular formula:C11H17NO4
- Molecular Weight:227.26
![(1R,5S,6r)-3-tert-butyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20210111/GIF/134575-37-4.gif)
134575-37-4
11 suppliers
inquiry
![(1R,5S,6R)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.1.0]hexane-6-carboxylic acid](/CAS/GIF/927679-54-7.gif)
927679-54-7
117 suppliers
$29.00/100mg
Yield:927679-54-7 96.5%
Reaction Conditions:
Stage #1:3-(tert-butyl) 6-ethyl (1R,5S,6r)-3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate with sodium hydroxide in ethanol at 0 - 20; for 3 h;
Stage #2: with hydrogenchloride in water
Steps:
3 3-(tert-Butoxycarbonyl)-3-azabicyclo[3.1.0]hexane-6-carboxylic acid
The tert-butyl 6-ethoxycarbonyl-3-azabicyclo[3.1.0]hexane-3-carboxylate exo-isomer (613 mg, 2.40 mmol) prepared in Example 2 was dissolved in 6 mL of ethanol and 2N NaOH (2.40 mL, 4.80 mmol) was added dropwise at 0° C. After stirring at room temperature for 3 hours, the completion of the reaction was confirmed by TLC (hexane:ethyl acetate=6:1). After the reaction was completed, the reaction mixture was concentrated under reduced pressure and then extracted by adding methylene chloride and water. After removing the organic layer containing byproducts, the aqueous layer was acidified with 1N HCl and extracted with methylene chloride. The organic layer was dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to obtain 527 mg (96.5%) of the target compound. 1H NMR (300 MHz, CDCl3) δ 3.74 (d, J=11.1 Hz, 1H), 3.66 (d, J=11.3 Hz, 1H), 3.53-3.45 (m, 2H), 2.17 (br, 2H), 1.55-1.53 (m, 1H), 1.48 (s, 9H).
References:
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY;NAM, Ghil Soo;CHOI, Kyung Il;PAE, Ae Nim;SEO, Seon Hee;CHOO, Hyun Ah;KEUM, Gyo Chang;KIM, Jung Hyun US2014/343295, 2014, A1 Location in patent:Paragraph 0087; 0088; 0089
![exo-3-Boc-3-azabicyclo[3.1.0]hexane-6-methanol](/CAS2/GIF/419572-18-2.gif)
419572-18-2
79 suppliers
$52.00/100mg
![(1R,5S,6R)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.1.0]hexane-6-carboxylic acid](/CAS/GIF/927679-54-7.gif)
927679-54-7
117 suppliers
$29.00/100mg
![3-Oxabicyclo[3.1.0]hexane-6-carboxylicacid,2,4-dioxo-,(1alpha,5alpha,6alpha)-(9CI)](/CAS/GIF/187681-87-4.gif)
187681-87-4
13 suppliers
inquiry
![(1R,5S,6R)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.1.0]hexane-6-carboxylic acid](/CAS/GIF/927679-54-7.gif)
927679-54-7
117 suppliers
$29.00/100mg
![ethyl 5-benzyl-1,3a,4,5,6,6a-hexahydro-4,6-dioxopyrrolo[3,4-c]pyrazole-3-carboxylate](/CAS/GIF/134575-05-6.gif)
134575-05-6
13 suppliers
inquiry
![(1R,5S,6R)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.1.0]hexane-6-carboxylic acid](/CAS/GIF/927679-54-7.gif)
927679-54-7
117 suppliers
$29.00/100mg
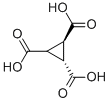
705-35-1
13 suppliers
$45.00/50mg
![(1R,5S,6R)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.1.0]hexane-6-carboxylic acid](/CAS/GIF/927679-54-7.gif)
927679-54-7
117 suppliers
$29.00/100mg