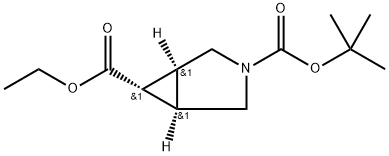
(1R,5S,6r)-3-tert-butyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate synthesis
- Product Name:(1R,5S,6r)-3-tert-butyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate
- CAS Number:134575-37-4
- Molecular formula:C13H21NO4
- Molecular Weight:255.31
![Ethyl 3-azabicyclo[3.1.0]hexane-6-carboxylate](/CAS/GIF/174456-77-0.gif)
174456-77-0
28 suppliers
$502.75/5MG

24424-99-5
840 suppliers
$13.50/25G
![(1R,5S,6r)-3-tert-butyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20210111/GIF/134575-37-4.gif)
134575-37-4
11 suppliers
inquiry
Yield:134575-37-4 82%
Reaction Conditions:
Stage #1: [(1α,5α,6α)-3-aza-bicyclo[3.1.0]hexane]-6-carboxylic acid ethyl esterwith triethylamine in dichloromethane at 0; for 0.333333 h;
Stage #2: di-tert-butyl dicarbonate in dichloromethane at 0 - 25; for 18 h;
Steps:
Ethyl (1 R,5S,6r)-3-azabicyclo[3.1 0]hexane-6-carboxylate (Intermediate 9) (2.0 g, 8.16 mmol) was dissolved in DCM (25 ml.) and triethylamine (3.45 ml_, 24.5 mmol) was added. The mixture was cooled to 0 °C, stirred for 20 mins and di-ferf-butyl dicarbonate (2.65 g, 12.2 mmol) was added. The resulting reaction mixture was then stirred at 25 °C for 18 h. The solvents were removed in-vacuo and the residue was partitioned between H20 (80 ml_) and ethyl acetate (60 mL). The aqueous layer was further extracted with ethyl acetate (2 c 60 mL), the combined organic layers were dried (Na2S04) and the solvent was removed in- vacuo. The residue was purified by column chromatography (Normal- Phase activated Al203, 0 % to 40 % ethyl acetate in hexanes) to give 3-(fe/f-butyl) 6-ethyl (1 R,5S,6r)- 3-azabicyclo[3.1 .0]hexane-3,6-dicarboxylate (1.70 g, 82 %) as a liquid. LCMS (System 3, Method F): m/z 241 (M-15+H)+ (ESI +ve), at 4.06 min, 202 nm.
References:
WO2019/243850,2019,A1 Location in patent:Page/Page column 61
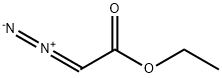
623-73-4
149 suppliers
$27.30/25ML
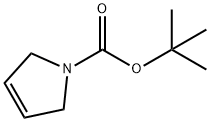
73286-70-1
288 suppliers
$6.00/5g
![Ethyl endo-3-Boc-3-azabicyclo-[3.1.0]hexane-6-carboxylate](/CAS/GIF/827599-20-2.gif)
827599-20-2
16 suppliers
$310.00/100mg
![(1R,5S,6r)-3-tert-butyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20210111/GIF/134575-37-4.gif)
134575-37-4
11 suppliers
inquiry

623-73-4
149 suppliers
$27.30/25ML

73286-70-1
288 suppliers
$6.00/5g
![(1R,5S,6r)-3-tert-butyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20210111/GIF/134575-37-4.gif)
134575-37-4
11 suppliers
inquiry

24424-99-5
840 suppliers
$13.50/25G
![Ethyl endo-3-Boc-3-azabicyclo-[3.1.0]hexane-6-carboxylate](/CAS/GIF/827599-20-2.gif)
827599-20-2
16 suppliers
$310.00/100mg
![(1R,5S,6r)-3-tert-butyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20210111/GIF/134575-37-4.gif)
134575-37-4
11 suppliers
inquiry