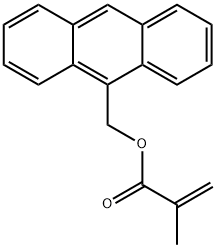
9-Anthracenylmethyl methacrylate synthesis
- Product Name:9-Anthracenylmethyl methacrylate
- CAS Number:31645-35-9
- Molecular formula:C19H16O2
- Molecular Weight:276.33
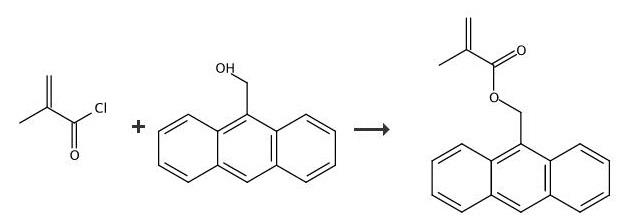
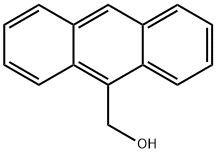
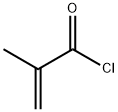
Experimental procedrure: 9-Anthracene-methanol 9.3g (45mmol), methylene chloride 60ml and methacryloyl chloride 8g (77mmol) were charged into a 300ml three-necked flask equipped with a stirrer and thermometer. Methylene chloride 10ml solution of triethylamine 6.0g (59mmol) was added dropwise while cooling the slurry with ice water. After completion of the dropwise addition, the slurry became a homogeneous solution. Then allowed to stand overnight while cooling in ice water. The hydrochloride salt of triethylamine floating on the liquid surface and water 30ml were sufficiently added. Further stirred to dissolve. Again washed with water 30ml, the obtained methylene chloride solution was dried over anhydrous sodium sulfate. Further methanol was added to the filtered methylene chloride solution 100ml, stored in the refrigerator and recrystallized. After 2 days, the precipitated crystals were suction filtered and dried. Pale yellow microcrystals 7.1g were obtained. Analysis results of the obtained substance are as shown below, this compound was confirmed to be 9-Anthracenylmethyl methacrylate by 1H-NMR, IR. Yield 58mol%. Melting point: 59-60 ° CIR (KBr, cm-1): 1715, 1624, 1450, 1320, 1296, 1164, 1150, 1060, 1008, 964, 946, 892, 740, 700, 640, 600, 568, 500. 1H-NMR (CDCl3, ppm): δ1.92 (s, 3H, methyl), 5.50 (s, 1H, vinyl), 6.05 (s, 1H, vinyl), 6.22 (s, 2H, methylene group), 7.44-7.60 (m, 4H, anthracene ring), 8.03 (d, J = 10Hz, 2H, anthracene ring), 8, 38 (d, J = 10Hz, 2H, anthracene ring), 8.51 (s, 1H, anthracene ring).
1468-95-7
360 suppliers
$8.00/5g
920-46-7
343 suppliers
$20.00/1g

31645-35-9
171 suppliers
$30.00/100mg
Yield:31645-35-9 90%
Reaction Conditions:
with triethylamine at 20;
Steps:
2.3. Synthesis of Fluorophore-ContainingMethacrylate Monomers (AntMA: 1 andPyMA: 2)
9-Anthracenemethanol (4.5 g; 0.022 mol) was addedto a solution of triethylamine (9.0 mL; 0.065 mol) in250 mL of anhydrous MC. Methacryloyl chloride (6.3 mL;0.065 mol) was added dropwise under stirring at 0 C.The reaction was then conducted at room temperatureovernight, after which the reaction medium was filtered.The solvent was evaporated, and the solid residue waspurified by recrystallization in 95% ethanol at 40 C(yield = 90%). 1H NMR (CDCl3, ppm: 7.45-8.50(m, 9H, aromatic H), 6.20 (s, 2H, CH2O), 6.05 (s, 1H,CH2= C), 5.56 (s, 1H, CH2= C), 1.97 (s, 3H, CH3 .
References:
You, Jungmok;Kim, Eunkyoung [Journal of Nanoscience and Nanotechnology,2016,vol. 16,# 10,p. 10927 - 10934]
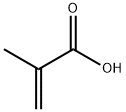
79-41-4
531 suppliers
$14.00/5g

1468-95-7
360 suppliers
$8.00/5g

31645-35-9
171 suppliers
$30.00/100mg