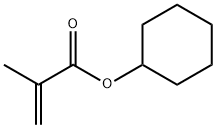
Cyclohexyl methacrylate synthesis
- Product Name:Cyclohexyl methacrylate
- CAS Number:101-43-9
- Molecular formula:C10H16O2
- Molecular Weight:168.23
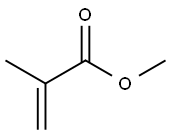
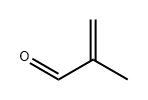
80-62-6
622 suppliers
$18.00/25mL
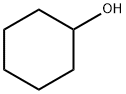
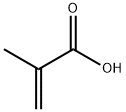
108-93-0
438 suppliers
$11.00/25g

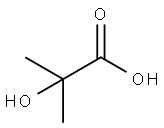
101-43-9
307 suppliers
$7.00/25g
Yield:101-43-9 93%
Reaction Conditions:
with C2H3O3(1-)*C37H78N(1+) in hexane at 90; for 2 h;Molecular sieve;
Steps:
64
General procedure: In Examples 62 to 64, as shown in Table 13, a transesterification reaction was carried out between methyl methacrylate which is an easily polymerizable ester compound and an alcohol compound. In Example 62, as in Example 24, lanthanum nitrate and tri n-octylphosphine were previously azeotropically refluxed with dimethyl carbonate for 1 hour, then dimethyl carbonate was evaporated at room temperature, The obtained catalyst was used. In Examples 63 and 64, methyltridodecylammonium methyl carbonate was used singly. As a result, ester products were obtained in high yield. It is to be noted that in Example 62, when using the isolated phosphonium salt as a catalyst instead of using the prepared catalyst as it is, it is predicted that the ester product is obtained with a higher yield than in Example 62 . Although not shown in Table 13, potassium tert-butoxide was used as a catalyst, and various by-products were produced.
References:
NAGOYA UNIVERSITY;MITSUBISHI RAYON COMPANY LIMITED;SHIHARA, KAZUAKI;HATANO, MANABU JP5804472, 2015, B2 Location in patent:Paragraph 0087; 0088