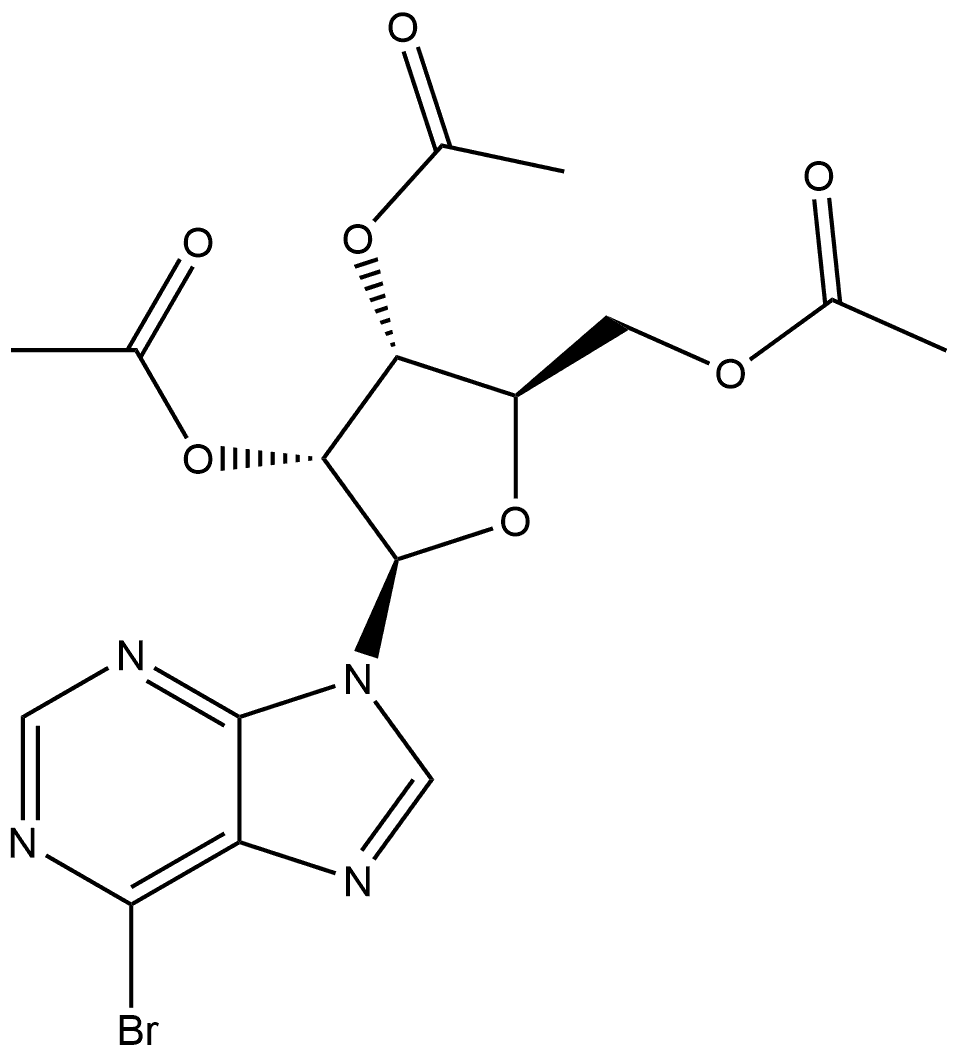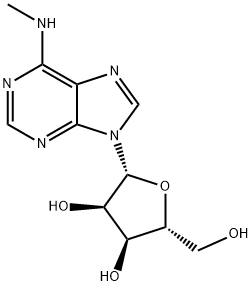
6-METHYLAMINOPURINE 9-RIBOFURANOSIDE synthesis
- Product Name:6-METHYLAMINOPURINE 9-RIBOFURANOSIDE
- CAS Number:1867-73-8
- Molecular formula:C11H15N5O4
- Molecular Weight:281.27
2004-06-0
214 suppliers
$8.00/1g
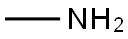
74-89-5
7 suppliers
$13.44/25ML

1867-73-8
149 suppliers
$32.00/250mg
Yield:1867-73-8 94%
Reaction Conditions:
in ethanol;water;Sealed tube;Reflux;
Steps:
N6-Methyladenosine
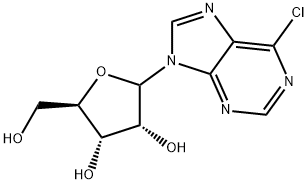
6-Chloro-9-(β-D-ribofuranosyl)purine (300 mg, 1.05 mmol) was suspended in an aqueous solution of 40% methylamine, sealed and stirred overnight. The mixture was evaporated under vacuum, and the residue was refluxed on stirring with 10 ml ethanol. After cooling and stirring for 4 h the precipitate was filtered withsuction and dried under high vacuum to give 277 mg (94%) of N6-methyladenosine as a colorless solid. 1HNMR (DMSO-d6) δ 8.33 (s, 1H), 8.22 (bs, 1H), 7.80 (bs, 1H), 5.88 (d, J = 6.2 Hz, 1H), 4.60 (dd, J = 6.0, 11.3Hz, 1H), 4.14 (m, 1H), 3.96 (dd, J = 3.4, 6.6 Hz, 1H), 3.68 (m, 1H), 3.55 (m, 1H), 3.31 (s, 3H); MS (ESI+): m/z282.1 [M+H]+; MS (ESI-): m/z 326.1 [M+formate]-; purity, 98.9%, RT 4.85 min (gradient from 0.1 to 50% ofMeCN in 0.1% aqueous formic acid for 15 min).
References:
Yanachkov, Ivan B.;Chang, Hung;Yanachkova, Milka I.;Dix, Edward J.;Berny-Lang, Michelle A.;Gremmel, Thomas;Michelson, Alan D.;Wright, George E.;Frelinger, Andrew L. [European Journal of Medicinal Chemistry,2016,vol. 107,art. no. 8190,p. 204 - 218] Location in patent:supporting information
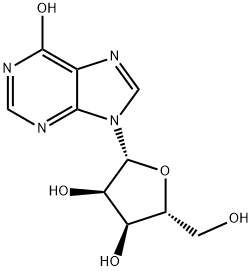
58-63-9
644 suppliers
$5.00/25g
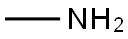
74-89-5
7 suppliers
$13.44/25ML

1867-73-8
149 suppliers
$32.00/250mg
![Adenosine, N-[(dimethylamino)methylene]-](/CAS/20210305/GIF/17331-15-6.gif)
17331-15-6
0 suppliers
inquiry

1867-73-8
149 suppliers
$32.00/250mg
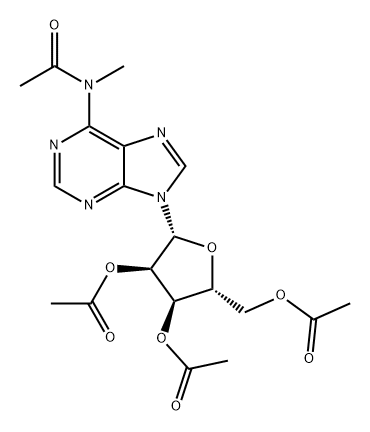
71118-24-6
0 suppliers
inquiry

1867-73-8
149 suppliers
$32.00/250mg