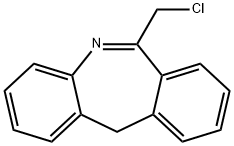
6-Chloromethylmorphanthridine synthesis
- Product Name:6-Chloromethylmorphanthridine
- CAS Number:21535-44-4
- Molecular formula:C15H12ClN
- Molecular Weight:241.72
![N-[2-(Phenylmethyl)phenyl]-2-chloroacetamide](/CAS/GIF/21535-43-3.gif)
21535-43-3
83 suppliers
$120.50/50 mg

21535-44-4
79 suppliers
inquiry
Yield: 95.8%
Reaction Conditions:
with sulfuric acid;lanthanum(lll) triflate in dimethyl sulfoxide;toluene at 90; for 2.5 h;Reagent/catalyst;Temperature;Solvent;
Steps:
2
26.0 g (0.10 mol) of N- [2- (phenylmethyl) phenyl] -2-chloroacetamide and 180 ml of toluene were added 500ml four-necked flask, add 1.0g (0.01mol) concentrated sulfuric acid, stirring, 2.93 g (0.005 mol) of lanthanum triflate and 1 ml of DMSO were added, the temperature was raised to 90 ° C., the mixture was incubated for 2.5 hours, Water is removed during the reaction. After the incubation was completed, about 75 ml of toluene was distilled off and the temperature was lowered to 5 ° C. Incubated for 2 hours, suction filtered, and dried at 40 ° C for 10h to obtain 23.1g of 6-chloromethylmorphanthridine, Purity 99.1%, yield 95.8%.
References:
Beijing Langyi Pharmaceutical Co., Ltd.;Xu Lei;Meng Bin;Sun Bin;Ma Qingshuang;Wang Xiaoguang;Nan Hongyan;Zhang Tong CN107286093, 2017, A Location in patent:Paragraph 0031; 0034-0035; 0038; 0041; 0043; 0045; 0047
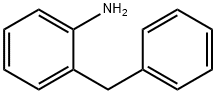
28059-64-5
193 suppliers
$6.00/1g

21535-44-4
79 suppliers
inquiry