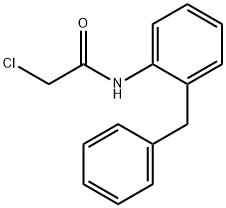
N-[2-(Phenylmethyl)phenyl]-2-chloroacetamide synthesis
- Product Name:N-[2-(Phenylmethyl)phenyl]-2-chloroacetamide
- CAS Number:21535-43-3
- Molecular formula:C15H14ClNO
- Molecular Weight:259.73
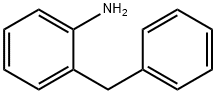
28059-64-5
193 suppliers
$6.00/1g
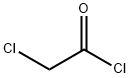
79-04-9
374 suppliers
$12.00/5g
![N-[2-(Phenylmethyl)phenyl]-2-chloroacetamide](/CAS/GIF/21535-43-3.gif)
21535-43-3
83 suppliers
$120.50/50 mg
Yield: 85%
Reaction Conditions:
with pyridine in toluene at 0 - 20; for 1.5 h;Inert atmosphere;
Steps:
N-(2-benzylphenyl)-2-chloroacetamide (12).
A samples of commercially available 2-benzylaniline (1841 mg, 10.05 mmol) in toluene (10 mL) at 0 °C under Ar was treated with chloroacetyl chloride (0.82 mL, 10.25 mmol) and pyridine (0.98 mL, 12.11 mmol). Reaction mixture was stirred at 0 °C for 30 min and at rt for 1 h, and the reaction was quenched with water (5 mL). Resulting mixture was stirred for 20 min, diluted with EtOAc (200 mL), washed with aqueous 1N HCl (3 x 30 mL) and saturated aqueous NaCl, dried over anhydrous MgSO4 and concentrated under reduced pressure to provide compound 12 as a gray solid (2205 mg, 85%): 1H NMR (CDCl3, 400 MHz) δ 7.99 (br s, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.34-7.11 (m, 8H), 4.06 (s, 2H), 4.00 (s, 2H); CAS Registry: 21535-43-3.
References:
Park, Sang Won;Kang, Han Eol;Yun, Wheesahng;Lee, Sang Yeul;Nam, Tae-gyu [Tetrahedron Letters,2019,art. no. 151451] Location in patent:supporting information