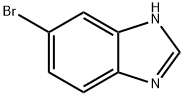
5-Bromo-1H-benzimidazole synthesis
- Product Name:5-Bromo-1H-benzimidazole
- CAS Number:4887-88-1
- Molecular formula:C7H5BrN2
- Molecular Weight:197.03
1575-37-7
373 suppliers
$10.00/1g
149-73-5
546 suppliers
$12.00/5g

4887-88-1
196 suppliers
$5.00/100mg
Yield:4887-88-1 100%
Reaction Conditions:
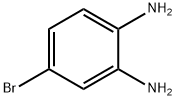
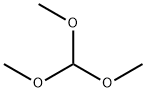
Stage #1:4-Bromo-benzene-1,2-diamine;trimethyl orthoformate with hydrogenchloride in water;N,N-dimethyl-formamide at 20; for 1 h;
Stage #2: with sodium hydrogencarbonate in water;N,N-dimethyl-formamide; pH=7
Steps:
87.87a
Step 87a: 7?rt-butyl 5-bromo-lH-benzo[d]imidazole-l-carboxylate (Compound 0601-187)To a solution of 4-bromobenzene-l,2-diamine (3 g, 16 mmol) in DMF (22 mL) were added trimethyl orthoformate (44 mL) and cone. HC1 (1.5 mL) and the mixture was stirred at room temperature for 1 h. The mixture was diluted with water (200 mL) and adjusted to pH7 with saturated aqueous NaHC03, extract with ethyl acetate (200 mL). The organic layer was dried over Na2S04, concentrated to give 5-bromo-lH-benzo[d]imidazole (3.25 g, 100%) as an off-white solid. LCMS: 197 [M+l]+. 1H NMR (400 MHz, DMSO-; = 7.6 Hz, J= 40 Hz, 1H), 7.79 (d, J= 47.2 Hz, 1H), 8.26 (s, 1H), 12.61 (d, J= 25.6 Hz, 1H).
References:
CURIS, INC.;BAO, Rudi;LAI, Chengjung;QIAN, Changgeng WO2011/130628, 2011, A1 Location in patent:Page/Page column 228
122-51-0
508 suppliers
$10.00/5ml

1575-37-7
373 suppliers
$10.00/1g

4887-88-1
196 suppliers
$5.00/100mg
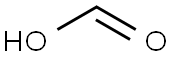
64-18-6
1115 suppliers
$22.12/250ML

1575-37-7
373 suppliers
$10.00/1g

4887-88-1
196 suppliers
$5.00/100mg
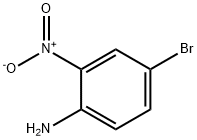
875-51-4
306 suppliers
$5.00/1g
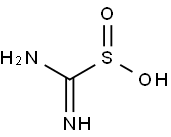
1758-73-2
462 suppliers
$18.00/25g

4887-88-1
196 suppliers
$5.00/100mg
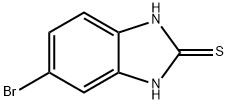
68468-39-3
48 suppliers
$197.00/1g

4887-88-1
196 suppliers
$5.00/100mg