
5-Aminoquinoline synthesis
- Product Name:5-Aminoquinoline
- CAS Number:611-34-7
- Molecular formula:C9H8N2
- Molecular Weight:144.17
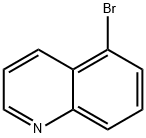
4964-71-0
305 suppliers
$6.00/1g

611-34-7
306 suppliers
$6.00/1g
Yield:611-34-7 89%
Reaction Conditions:
with ammonium hydroxide;2-O-methyl-L-inositol;copper(II) acetate monohydrate in 1-methyl-pyrrolidin-2-one at 110; for 12 h;
Steps:
Synthesis of 5a-z, 5aa; General Procedure C
General procedure: To a sealed reaction vessel were added ammonia (aq, 25%) (1.8 mL, 12.8 mmol),Cu(OAc)2·H2O (0.13 mmol), QCT (0.26 mmol) in NMP (1.8 mL) and aryl halides(1.28 mmol). The resulting mixture was heated in an oil bath with appropriatetemperature under rapid stirring for the indicated time. After complete consumption ofthe aryl halide as monitored by TLC, the reaction vessel was cooled to r.t. It was openedto air, and the reaction mixture was extracted with ethyl acetate (3×10 mL), and theorganic layer was washed with water (2×10 mL) and once with brine (10 mL), driedwith magnesium sulfate and concentrated in vacuo. The product was purified bycolumn chromatography on silica gel.
References:
Chen, Guoliang;Chen, Yuanguang;Du, Fangyu;Fu, Yang;Wu, Ying;Zhou, Qifan [Synlett,2019,vol. 30,# 19,p. 2161 - 2168] Location in patent:supporting information
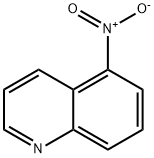
607-34-1
228 suppliers
$14.00/1g

611-34-7
306 suppliers
$6.00/1g

607-34-1
228 suppliers
$14.00/1g
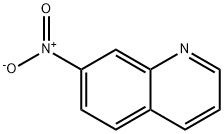
613-51-4
125 suppliers
$8.00/250mg
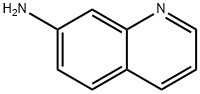
580-19-8
135 suppliers
$15.00/250mg

611-34-7
306 suppliers
$6.00/1g
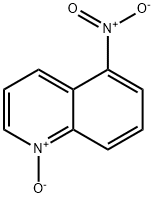
7613-19-6
15 suppliers
inquiry

611-34-7
306 suppliers
$6.00/1g
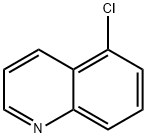
635-27-8
142 suppliers
$7.00/100mg

611-34-7
306 suppliers
$6.00/1g