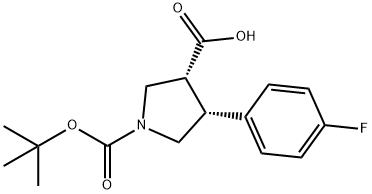
4-(4-FLUORO-PHENYL)-PYRROLIDINE-1,3-DICARBOXYLIC ACID 1-TERT-BUTYL ESTER synthesis
- Product Name:4-(4-FLUORO-PHENYL)-PYRROLIDINE-1,3-DICARBOXYLIC ACID 1-TERT-BUTYL ESTER
- CAS Number:455954-94-6
- Molecular formula:C16H20FNO4
- Molecular Weight:309.33

24424-99-5
832 suppliers
$13.50/25G

455954-94-6
22 suppliers
$80.00/250mg
Yield:455954-94-6 91%
Reaction Conditions:
Stage #1: (+)-4-(4-fluoro-phenyl)-pyrrolidine-1,3-dicarboxylic acid 1-tert-butyl ester 3-methyl esterwith hydrogenchloride;water for 4 h;Heating / reflux;
Stage #2: di-tert-butyl dicarbonatewith sodium hydroxide in 1,4-dioxane;water at 0 - 20; pH=8;
Stage #3: with hydrogenchloride in water at 0; pH=1;
Steps:
d
A mixture of 1.79 g (5.54 mmol) (+)-4-(4-fluoro-phenyl)-pyrrolidine-1,3-dicarboxylic acid 1-tert-butyl ester 3-methyl ester and 15 ml of a 2 M aqueous hydrochloric acid solution was heated at reflux for 4 h. The reaction mixture was cooled to 0° C. and basified by the addition of 3.6 ml of a 32% aqueous solution of sodium hydroxide. Dilution with 10 ml 1,4-dioxane was followed by addition of a solution of 2.42 g (11.1 mmol) di-tert.-butyl dicarbonate in 5 ml 1,4-dioxane. After completed addition the reaction mixture was allowed to slowly warm to room temperature over night. The mixture was extracted methyl tert.-butyl ether at pH 8. The organic layer was extracted with a 1 M aqueous sodium hydroxide solution. The combined aqueous layers were acidified to pH 1 by the addition of and ice cold 2 M aqueous hydrochloric acid solution at 0° C. and extracted with three portions of ethyl acetate. The combined organic layers were dried over sodium sulfate, concentrated in vacuo and purified by flash chromatography to give 1.55 g (91%) of the title compound as an off-white solid. MS m/e (%): 308 ([M-H+]-, 100) [α]D=+31.32 (c=0.3768, CHCl3)
References:
US2007/129419,2007,A1 Location in patent:Page/Page column 13-14