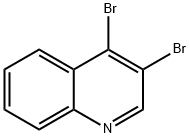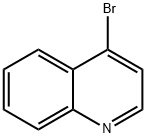
4-Bromoquinoline synthesis
- Product Name:4-Bromoquinoline
- CAS Number:3964-04-3
- Molecular formula:C9H6BrN
- Molecular Weight:208.05
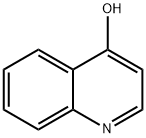
611-36-9
394 suppliers
$8.00/5g

3964-04-3
186 suppliers
$26.39/1g
Yield:3964-04-3 88%
Reaction Conditions:
with phosphorus tribromide in N,N-dimethyl-formamide; for 0.666667 h;Inert atmosphere;
Steps:
1.1 Example 1 - Synthesis of Compound 1 (4-Phenylethynyl-quinoline)
Step 1 : To a stirred solution of quinolin-4-ol (4.00 g, 27.6 mmol) in dry DMF (30 mL) was added phosphorus tribromide (7.61 g, 28.2 mmol) dropwise for 10 min. The reddish colored suspension was stirred for 30 min under nitrogen atmosphere. After complete consumption of starting material, the reaction mixture was quenched with ice, stirred for anther 30 min, then basified to pH -10 with a solution of saturated sodium bicarbonate (20 mL). The reaction mixture was extracted with ethyl acetate (2 x 100 mL) and the combined organic phase was dried over anhydrous sodium sulfate, filtered and evaporated in vacuo. The residue was purified on silica gel column using dichloromethane / methanol (0% to 10%) as eluent to give 4-bromo- quinoline (5.04 g; 88%) as a yellow solid; LCMS (ESI) 208 (M+H); H NMR (400 MHz, CHLOROFORM-d) δ ppm: 8.69 (d, J=4.69 Hz, 1 H), 8.20 (dd, J=8 39, 0.88 Hz, 1 H), 8.08 - 8.16 (m, 1 H), 7.78 (ddd, J=8.39, 6.98, 1 .37 Hz, 1 H), 7.71 (d, J=4.69 Hz, 1 H), 7.66 (ddd, J=8.31 , 7.04, 1.15 Hz, 1 H).
References:
WO2014/121883,2014,A1 Location in patent:Page/Page column 51
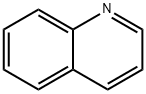
91-22-5
407 suppliers
$10.00/25g

3964-04-3
186 suppliers
$26.39/1g
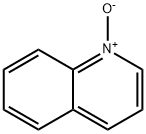
1613-37-2
0 suppliers
$54.31/5G
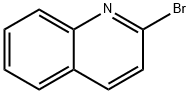
2005-43-8
211 suppliers
$6.00/250mg

3964-04-3
186 suppliers
$26.39/1g
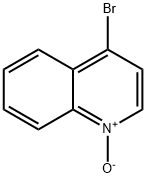
22614-98-8
9 suppliers
inquiry

3964-04-3
186 suppliers
$26.39/1g