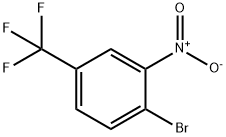
4-Bromo-3-nitrobenzotrifluoride synthesis
- Product Name:4-Bromo-3-nitrobenzotrifluoride
- CAS Number:349-03-1
- Molecular formula:C7H3BrF3NO2
- Molecular Weight:270
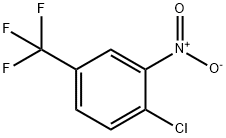
121-17-5
17 suppliers
$19.00/250g

349-03-1
212 suppliers
$8.00/5g
Yield:349-03-1 38%
Reaction Conditions:
with copper(I) bromide;lithium bromide in tetrahydrofuran at 160; for 6 h;Product distribution / selectivity;
Steps:
11
The above reaction was repeated but using acetic acid, acetonitrile or tetrahydrofuran instead of benzonitrile at various concentrations (0.02, 0.04, 0.1 and 1 ml/mmol). Table 14 shows the results, from which it may be seen that the optimal concentration depends upon the solvent, and that the best results were obtained at high concentration.
References:
Bayer CropScience S.A. US6635780, 2003, B1 Location in patent:Page/Page column 12
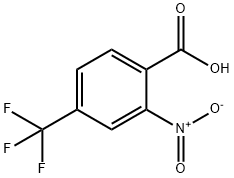
320-94-5
232 suppliers
$9.00/1g

349-03-1
212 suppliers
$8.00/5g
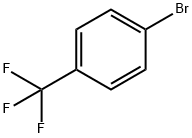
402-43-7
441 suppliers
$6.00/10g

349-03-1
212 suppliers
$8.00/5g