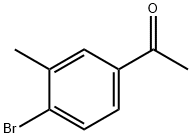
4'-Bromo-3'-methylacetophenone synthesis
- Product Name:4'-Bromo-3'-methylacetophenone
- CAS Number:37074-40-1
- Molecular formula:C9H9BrO
- Molecular Weight:213.07
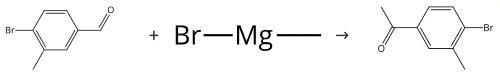 Figure Synthesis of 4'-Bromo-3'-methylacetophenone
Figure Synthesis of 4'-Bromo-3'-methylacetophenone
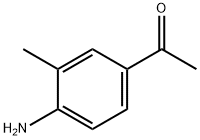
43230-11-1
10 suppliers
inquiry

37074-40-1
109 suppliers
$51.00/250mg
Yield: 84%
Reaction Conditions:
Stage #1:4'-amino-3'-methyl-acetophenone with sulfuric acid;sodium nitrite in acetonitrile at 0;
Stage #2: with urea in water;acetonitrile
Stage #3: with hydrogen bromide;copper(I) bromide in water;acetonitrile at 50; for 2 h;
Steps:
Step 2: Synthesis of 4'-bromo-3'-methylacetophenone 27.0 g of acetonitrile was added to a sulfuric acid solution of 27.0 g (181 mmol) of 4-acetyl-2-methylaniline, and the mixture was cooled to 0°C. An aqueous solution in which 13.1 g (190 mmol) of sodium nitrite was dissolved into 26.2 g of water was added in dropwise to the mixture. After reacting the resultant mixture for 1 hour at 0°C, an aqueous solution in which 1.1 g (18 mmol) of urea was dissolved into 2.2 g of water was added in dropwise, and the mixture was further stirred for 30 minutes to obtain an aqueous solution of 4-acetyl-2-methylbenzenediazonium sulfate. 5.18 g (36 mmol) of copper bromide, 62.2 g (362 mmol) of 47% hydrobromic acid and 81.0 g of acetonitrile were fed into another reactor. The aqueous solution of 4-acetyl-2-methylbenzenediazonium sulfate was added to this mixture in dropwise over 1 hour with stirring at 50°C. After adding in dropwise, the mixture was reacted for 1 hour at 50°C. Then, 81 g of toluene was added and the mixture was stirred for 30 minutes, and the water phase was separated. 54 g of toluene was added to the water phase and extracted again. The combined toluene solution was washed twice with 54 g of 14% aqueous ammonia and once with 54 g of water to obtain a toluene solution of 4'-bromo-3'-methylacetophenone. After removing the solvent by distilling under reduced pressure, the residue was distilled under reduced pressure of 1.5 kPa. 32.8 g of the obtained fraction which was collected in the range of outflow gas temperature from 130°C to 137°C was analyzed by HPLC. A percentage of relative area of 4'-bromo-3'-methylacetophenone was 99.1 % (yield 84%).
References:
Nissan Chemical Industries, Ltd. EP2172462, 2010, A1 Location in patent:Page/Page column 73-74
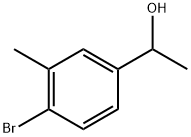
51930-77-9
3 suppliers
inquiry

37074-40-1
109 suppliers
$51.00/250mg
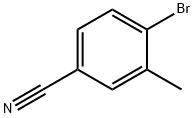
41963-20-6
178 suppliers
$15.00/1g
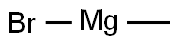
75-16-1
270 suppliers
$12.00/10ml

37074-40-1
109 suppliers
$51.00/250mg

95-46-5
300 suppliers
$10.00/10 g
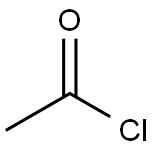
75-36-5
579 suppliers
$17.92/100G

37074-40-1
109 suppliers
$51.00/250mg
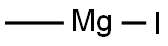
917-64-6
148 suppliers
$45.00/10ml

41963-20-6
178 suppliers
$15.00/1g

37074-40-1
109 suppliers
$51.00/250mg