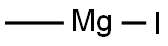
METHYLMAGNESIUM IODIDE synthesis
- Product Name:METHYLMAGNESIUM IODIDE
- CAS Number:917-64-6
- Molecular formula:CH3IMg
- Molecular Weight:166.24

74-88-4
351 suppliers
$15.00/10g
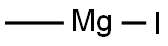
917-64-6
147 suppliers
$45.00/10ml
Yield:917-64-6 94%
Reaction Conditions:
with magnesium in diethyl ether at 25; under 5171.62 Torr;Inert atmosphere;Flow reactor;
Steps:
1.2.3. Grignard reagents (RMgX)
General procedure: Bromoethane (3.336 g, 2.28 mL, 30.00 mmol, 1 equiv) is dissolved in THF (22.7 mL) in a flask under argon. The organohalide 1.2 M solution is flowed through activated magnesium (chips/powder 1:1 wt %, 1.49 g, 60 mmol, 2 equiv) with BPR (100 psi) at 0.5 mL/min and 25 °C (Figure S1). After ≈4 min, the outcome solution is collected in a vial containing 2-hydroxybenzaldehyde phenylhydrazone (20-40 mg). When the yellow color solution turns orange, Grignard reagent solution is collected in a flask under argon. When the starting material solution is consumed, organomagnesium collection is maintained during 4 min (≈2-fold the residence time), pumping THF at 0.5 mL/min, yielding 90% of ethylmagnesium bromide as clear 1.08 M solution (≈20 mL, the yield is calculated in a steady state because the discarded volume of head and tail solution is constant, independently of the volume of organohalide solution converted). The concentration determination with 2-hydroxybenzaldehyde phenylhydrazone (82.3 mg, 0.38 mmol) dissolved in THF (1 mL) consumed 0.37 mL of EtMgBr corresponding to 1.03 M. The concentration determination with benzoic acid (46.2 mg, 0.37 mmol) and 4-(phenylazo)diphenylamine (indicator) dissolved in THF (1 mL) consumed 0.33 mL of EtMgBr corresponding to 1.12 M.
References:
Adamo, Andrea;Berton, Mateo;McQuade, D. Tyler;Sheehan, Kevin [Beilstein Journal of Organic Chemistry,2020,vol. 16,p. 1343 - 1356] Location in patent:supporting information
7439-95-4
0 suppliers
$11.60/0.9g

74-88-4
351 suppliers
$15.00/10g
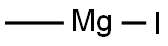
917-64-6
147 suppliers
$45.00/10ml
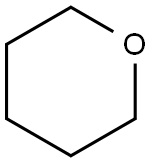
142-68-7
191 suppliers
$13.00/25mL
917-64-6
147 suppliers
$45.00/10ml