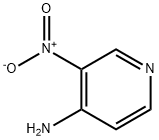
4-Amino-3-nitropyridine synthesis
- Product Name:4-Amino-3-nitropyridine
- CAS Number:1681-37-4
- Molecular formula:C5H5N3O2
- Molecular Weight:139.11
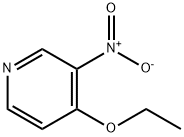
1796-84-5
166 suppliers
$9.00/250mg

1681-37-4
342 suppliers
$5.00/1g
Yield:1681-37-4 75%
Reaction Conditions:
with ammonium acetate;triethylamine in phosphorus pentaoxide;ethyl acetate
Steps:
2 Preparation of 3-Nitro-4-aminopyridine
EXAMPLE 2 Preparation of 3-Nitro-4-aminopyridine A 25 ml round bottom flask was fitted with a reflux condenser and magnetic stirrer and placed in an oil bath. The flask was charged with 1.0 g (5.95 mmol) of 4-ethoxy-3-nitropyridine and 5.0 g (65 mmol) of ammonium acetate. The reaction mixture was heated at 120° C. to provide a homogeneous liquid. The progress of the reaction was followed by thin layer chromotography employing a 10:1 ethyl acetate:triethylamine solvent system. After 21/2 hours the reaction mixture was cooled and poured into water. The yellow precipitate was collected by filtration, washed with water and dried in vacuo at 60° C. over phosphorus pentoxide. A total of 620 mg of 3-nitro-4-aminopyridine was obtained and chromatographically verified by comparison to an authentic reference standard. Yield 75%.
References:
Eli Lilly and Company US4552967, 1985, A
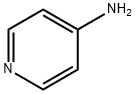
504-24-5
486 suppliers
$10.00/1 g

1681-37-4
342 suppliers
$5.00/1g
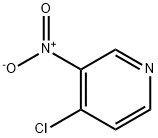
13091-23-1
349 suppliers
$14.14/1gm:

1681-37-4
342 suppliers
$5.00/1g
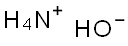
1336-21-6
730 suppliers
$14.56/250ML
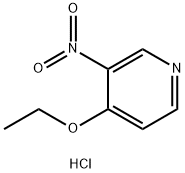
94602-04-7
70 suppliers
$37.79/5g

1681-37-4
342 suppliers
$5.00/1g

94602-04-7
70 suppliers
$37.79/5g

1681-37-4
342 suppliers
$5.00/1g