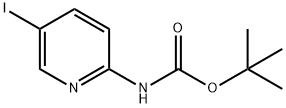
(5-IODO-PYRIDIN-2-YL)-CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:(5-IODO-PYRIDIN-2-YL)-CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:375853-79-5
- Molecular formula:C10H13IN2O2
- Molecular Weight:320.13
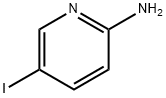
20511-12-0
464 suppliers
$6.00/1g
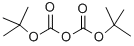
24424-99-5
864 suppliers
$13.50/25G

375853-79-5
64 suppliers
$45.00/50mg
Yield:375853-79-5 81%
Reaction Conditions:
Stage #1: 2-amino-5-iodopyridine;di-tert-butyl dicarbonatewith lithium hexamethyldisilazane in tetrahydrofuran at 0 - 20; for 16 h;
Stage #2: with ammonium chloride in tetrahydrofuran;water;
Steps:
A.i
Preparation of [5-(4-[1,2,3]triazol-1-yl-butyl)-pyridin-2-yl]-carbamic acid tert-butyl ester i) (5-Iodo-pyridin-2-yl)-carbamic acid tert-butyl ester A solution of 26.72 mL of a 1 M solution of bis-trimethylsilyl lithiumamide in THF was added to a solution of 5-iodo-pyridin-2-ylamine (3 g, 13.36 mmol) in anhydrous THF (30 ml) at 0° C. After warming to r.t. a solution of di-tert-butyl dicarbonate (2.77 g, 12.69 mmol) in THF was added and stirring continued for 16 h. The mixture was quenched with ammonium chloride solution and extracted with ethyl acetate. The extract was washed with brine, dried over Na2SO4 and concentrated. The crude product was washed with diethyl ether yielding (5-iodo-pyridin-2-yl)-carbamic acid tert-butyl ester as light orange solid. Yield 3.45 g, (81%) MS: M=264.7 (ESI+, M-tBu) 1H-NMR(400 MHz, CDCl3): δ=1.55 (s, 9H), 7.84 (d, 1H), 7.90 (d, 1H), 8.47 (s, 1H), 8.49 (s, 1H)
References:
US2006/63812,2006,A1 Location in patent:Page/Page column 16
