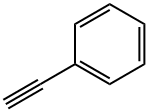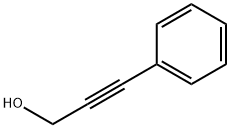
3-PHENYL-2-PROPYN-1-OL synthesis
- Product Name:3-PHENYL-2-PROPYN-1-OL
- CAS Number:1504-58-1
- Molecular formula:C9H8O
- Molecular Weight:132.16
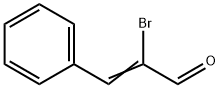
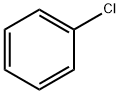
5443-49-2
181 suppliers
$40.29/25g

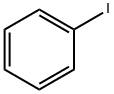
1504-58-1
192 suppliers
$8.00/1g
Yield:1504-58-1 75%
Reaction Conditions:
Stage #1:α-bromocinnamaldehyde with Triethoxysilane;[cis-Fe(H)(SPh)(PMe3)4] in tetrahydrofuran at 50; for 4 h;
Stage #2: with methanol;sodium hydroxide in tetrahydrofuran;water at 60; for 24 h;
Steps:
2.2. General procedure for catalytic hydrosilylation of aldehydes
General procedure: To a 25 mL Schlenk tube containing a solution of 1 in 2 mL of THF was added an aldehyde (1.0 mmol) and (EtO)3 SiH (0.20 g, 1.2 mmol). The reaction mixture was stirred at 50-55 °C until there was no aldehyde left (monitored by TLC and GC-MS). The reaction was then quenched byMeOH (2mL) and a 10% aqueous solution of NaOH (5 mL) with vigorous stirring at 60 °C for about 24 h.The organic product was extracted with diethyl ether (10 mL × 3), dried over anhydrous MgSO4, and concentrated under vacuum. The alcohol product was further purified using flash column chromatography (elute with 5-10% ethyl acetate in petroleum ether). The 1H NMR and 13C NMR spectra of the alcohol products are providedin Supporting information.
References:
Xue, Benjing;Sun, Hongjian;Niu, Qingfen;Li, Xiaoyan;Fuhr, Olaf;Fenske, Dieter [Catalysis Communications,2017,vol. 94,p. 23 - 28]