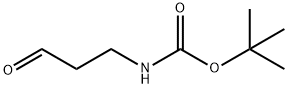
(3-OXO-PROPYL)-CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:(3-OXO-PROPYL)-CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:58885-60-2
- Molecular formula:C8H15NO3
- Molecular Weight:173.21
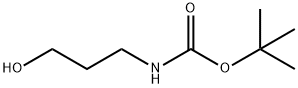
58885-58-8
195 suppliers
$10.00/1g

58885-60-2
90 suppliers
$117.00/50mg
Yield:58885-60-2 100%
Reaction Conditions:
with N-chloro-succinimide;2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;tetrabutyl-ammonium chloride;sodium hydrogencarbonate;potassium carbonate in chloroform;water for 2 h;
Steps:
A-2 A-2-Synthesis of N-BOC-3-aminopropanal (Compound 2);
A-2-Synthesis of N-BOC-3-aminopropanal (Compound 2); 18 g (103 mmol) of Compound 1, 1.62 g (10.4 mmol) of TEMPO (tetramethyl-1-piperidinyloxy, free radical), 2.9 g (10.45 mmol) of tetrabutylammonium chloride and 21 g (75.5 mmol) of N-chlorosuccinimide are suspended in 351 ml of NaHCO3/K2CO3 (0.5N/0.05N) and 351 ml of CHCl3. The reaction mixture is vigorously stirred for 2 hours. The organic phase is separated by settling, dried over MgSO4 and then concentrated on a rotary evaporator to give the expected aldehyde in the form of a light orange oil. Yield: 100% 1H NMR (CDCl3): 1.35 (s, 9H); 2.44 (d, 2H, J=6.8 Hz); 3.21 (m, 2H); 4.90 (broad s, 1H); 6.04 (dd, 1H, J=8 and 15.6 Hz); 6.74 (td, 1H, J=6.8 and 15.6 Hz); 9.39 (d, 1H, J=8 Hz).
References:
Laboratoire L. Lafon US6809096, 2004, B1 Location in patent:Page column 7
153815-24-8
1 suppliers
inquiry

58885-60-2
90 suppliers
$117.00/50mg
![tert-butyl N-[3-[methoxy(methyl)amino]-3-oxopropyl]carbamate](/CAS/20180703/GIF/142570-56-7.gif)
142570-56-7
34 suppliers
$45.00/50mg

58885-60-2
90 suppliers
$117.00/50mg
42116-55-2
56 suppliers
$60.00/50mg

58885-60-2
90 suppliers
$117.00/50mg

24424-99-5
861 suppliers
$13.50/25G

58885-60-2
90 suppliers
$117.00/50mg