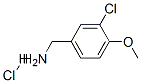
3-CHLORO-4-METHOXYBENZYLAMINE HYDROCHLORIDE synthesis
- Product Name:3-CHLORO-4-METHOXYBENZYLAMINE HYDROCHLORIDE
- CAS Number:41965-95-1
- Molecular formula:C8H11Cl2NO
- Molecular Weight:208.09
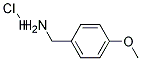
17061-61-9
10 suppliers
inquiry

41965-95-1
269 suppliers
$15.00/1 g
Yield:41965-95-1 26.5 g
Reaction Conditions:
with hydrogenchloride;dihydrogen peroxide in water at 63; for 11 h;
Steps:
2 Synthesis of First CMBA-HCl-Containing Composition
A four-neck 200 mL flask provided with a thermometer, Dimroth condenser and stirrer was charged with 35 g of the wet crystals of the MBA-HCl-containing composition having a purity of 99.7% obtained in Example 1 (mass in terms of dried product: 30.8 g (0.177 mol)), 153.3 g of water was added thereto, and they were stirred. Then, 46.1 g of 33.7% hydrochloric acid (theoretical molar ratio to MBA-HCl: 2.4) was dropped into the four-neck flask under the ordinary temperature for 30 minutes. After the dropping, the inner temperature was increased to 63° C. and 25.6 g of 33.6% hydrogen peroxide water (theoretical molar ratio to MBA-HCl: 1.43) was dropped into the four-neck flask for 30 minutes while maintaining the temperature. The reaction was carried out at 63° C. for 10 hours and the heating was finished when the remaining amount of MBA-based substances was confirmed to be 0.5% or less by the HPLC analysis. The reaction liquid was gradually cooled (about 5-15° C./hour) to carry out crystallization. Precipitation of crystals was confirmed at 48° C. and the state of a liquid temperature of 48° C. was therefore held for 1 hour. The cooling was continued at a cooling rate of 5-15° C./hour to 5° C. and the state of a liquid temperature of 5° C. was held for 1 hour. The reaction liquid after being held was filtrated to obtain a wet cake, which was washed with 48.7 g of isopropyl alcohol cooled to 5° C., and 31.4 g of wet crystals of a first CMBA-HCl-containing composition were obtained. The obtained wet crystals were dried using a vacuum drier and a dried product of 26.5 g of white crystals was obtained as the first CMBA-HCl-containing composition. The yield was 71.1 mol % and the HPLC purity was 99.1%. In the CMBA-HCl-containing composition, 0.08% of MBA-based substances remained and 0.84% of dichloro products were generated as by-products.
References:
AIR WATER INC.;FUJIMOTO, Takashi;YOKOTA, Keiichi;IDE, Takahiro US2017/44091, 2017, A1 Location in patent:Paragraph 0054
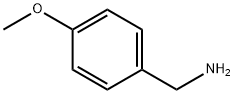
2393-23-9
484 suppliers
$20.00/25g

41965-95-1
269 suppliers
$15.00/1 g
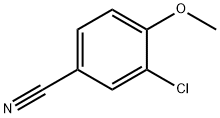
102151-33-7
102 suppliers
$7.00/1g

41965-95-1
269 suppliers
$15.00/1 g
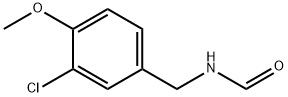
212576-67-5
6 suppliers
inquiry

41965-95-1
269 suppliers
$15.00/1 g
