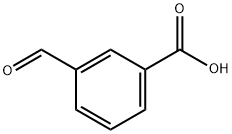
3-Carboxybenzaldehyde synthesis
- Product Name:3-Carboxybenzaldehyde
- CAS Number:619-21-6
- Molecular formula:C8H6O3
- Molecular Weight:150.13
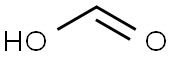
64-18-6
1084 suppliers
$22.12/250ML
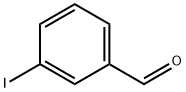
618-51-9
384 suppliers
$9.00/10g

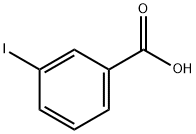
619-21-6
224 suppliers
$11.00/1g
Yield:619-21-6 81%
Reaction Conditions:
with iodine;triethylamine;triphenylphosphine in toluene at 80;Inert atmosphere;Sealed tube;
Steps:
Heterogeneous Palladium-Catalyzed Formylation of Aryl Iodides with HCOOH; General Procedure
General procedure: A dried 10 mL reaction tube was charged with I 2 (152 mg, 1.2 mmol),PPh 3 (315 mg, 1.2 mmol), and toluene (4 mL) under argon. The mixture was stirred at r.t. for 10 min. Then aryl iodide 1 (1 mmol), 2P-Fe3O4SiO2-Pd(OAc)2 (79 mg, 3 mol%), and Et3N (606 mg, 6 mmol)were added to this solution. After the addition of HCOOH (184 mg, 4mmol), the reaction tube was immediately sealed and the reactionmixture was stirred at 80°C for 3-5 h. After cooling to r.t., the Pd catalyst was magnetically separated from the mixture, washed with toluene (2 mL), distilled H2O (2 × 2 mL) and EtOH (2 × 2 mL), dried undervacuum at 80 °C, and used directly in the next cycle. The reaction mixture was then filtered and concentrated under vacuum. The residue was purified by silica gel column chromatography (light PE/EtOAc10:1) to afford the desired product 2.
References:
You, Shengyong;Zhang, Rongli;Cai, Mingzhong [Synthesis,2021,vol. 53,# 11,p. 1962 - 1970]
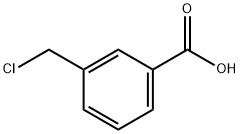
31719-77-4
216 suppliers
$11.40/0.5g

619-21-6
224 suppliers
$11.00/1g
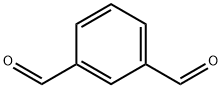
626-19-7
414 suppliers
$5.00/5g

619-21-6
224 suppliers
$11.00/1g

124-38-9
129 suppliers
$175.00/23402
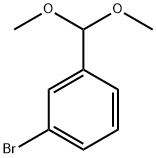
67073-72-7
26 suppliers
inquiry

619-21-6
224 suppliers
$11.00/1g