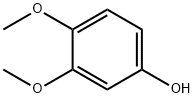
3,4-Dimethoxyphenol synthesis
- Product Name:3,4-Dimethoxyphenol
- CAS Number:2033-89-8
- Molecular formula:C8H10O3
- Molecular Weight:154.16
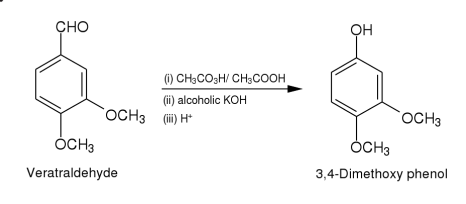
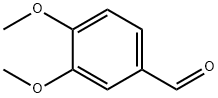
Method (i): To a solution of vertraldehyde (2.11)(5 g,0.03 mole)in glacial acetic acid (30 ml)is added dropwise to a solution of peracetic acid5(15 ml) during 30 min.The temperature of the reaction mixture rises,it is kept at 40-45 by cooling.The reaction mixture is left for 10 hr and then concentrated to about 15 ml in vacuo.The residue is extracted with ether (2 x 20 ml).The ether layer is distilled.The formate ester of 3,4- dimethoxyphenol thus obtained is hydrolysed by refluxing with potassium hydroxide (10 g)in aqueous alcohol (1:4,100 ml)for 1 hr.The reaction mixture is concentrated in vacuo almost to dryness.It is dissolved in water (20 ml),the solution rendered acidic with dilute sulphuric acid and extracted with ether.The ether extract is dried (sodium sulphate)and distilled.The oily residue thus obtained is subjected to column chromatography over silica gel(2 x 30 cm).Elution with benzene gave 3,4-dimethoxyphenol as yellow solid.Yield 3g(65.2%).It is crystallised from benzene as yellow crystalline needles.M.p.78-80(lit.m.p.78-80).
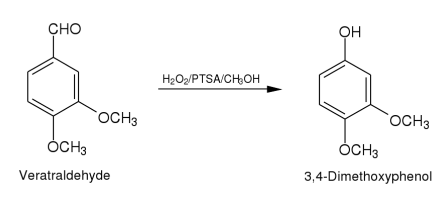
Method (ii): A mixture of veratraldehyde (2.11)(1.66 g,0.01 mole),hydrogen peroxide (30%,2.6 ml,0.023 mole),p-toluenesulphonic acid (6.88 g, 0.036 mole)and methanol (5 ml)is stirred at room temperature.When the reaction is complete (as monitored by TLC)the reaction mixture is diluted with water (50 ml)and extracted with dichloromethane (5 x 50 ml).The extract is dried (anhydrous sodium sulphate)and purified by column chromatography over silica gel(2 x 30 cm).Elution with benzene gave 3,4. dimethoxyphenol as an yellow solid.Yield 1.16 g (75.3%).M.p.78-80.
120-14-9
794 suppliers
$5.00/25g

2033-89-8
259 suppliers
$15.00/1g
Yield:2033-89-8 96%
Reaction Conditions:
Stage #1:3,4-dimethoxy-benzaldehyde with 3-chloro-benzenecarboperoxoic acid in dichloromethane at 20; for 15 h;
Stage #2: with potassium carbonate in methanol at 20; for 0.5 h;
Steps:
3,4-dimethoxyphenol (Compound 3)
Chloroperbenzoic acid (2.23 g with 77%, ca. 9.93 mmol) was added to a solution of 3,4-dimethoxybenzaldehyde (Compound 2) (1.50 g, 9.03 mmol) dissolved in CH2Cl2 (25 mL) Was stirred at room temperature for 15 hours. The reaction mixture was quenched by the addition of dimethyl sulfide (1 mL) and washed with a saturated aqueous solution of Na2SO3 (3 x 20 mL) and brine (20 mL). The organic solvent phase was dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was dissolved in methanol (23 mL) and then treated with K2CO3 (2.49 g, 18.05 mmol) and the mixture was stirred at room temperature for 30 min. After removal of the methanol, the residue was dissolved in EtOAc (30 mL), washed with H2O (2 x 15 mL), brine (15 mL) and dried over anhydrous Na2SO4. The organic solvent phase was filtered and concentrated, and the residue was purified by column chromatography (EtOAc / hexane = 1/1) to give a white solid compound 3 (1.34 g, 96%).
References:
Hallym University Industry-Academic Cooperation Foundation;Jeon, Chung Gab;Kongara, Damodar KR101740915, 2017, B1 Location in patent:Paragraph 0053-0054
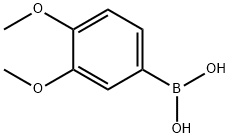
122775-35-3
332 suppliers
$5.00/1g

2033-89-8
259 suppliers
$15.00/1g
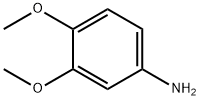
6315-89-5
324 suppliers
$7.00/10g

2033-89-8
259 suppliers
$15.00/1g
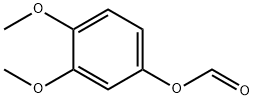
2033-88-7
0 suppliers
inquiry

2033-89-8
259 suppliers
$15.00/1g

67-56-1
776 suppliers
$7.29/5ml-f
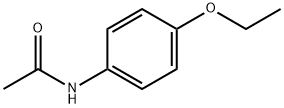
62-44-2
518 suppliers
$19.00/25g

2033-89-8
259 suppliers
$15.00/1g
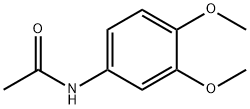
881-70-9
34 suppliers
$105.00/500mg
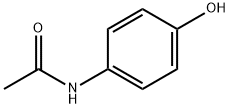
103-90-2
1074 suppliers
$9.00/1g