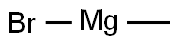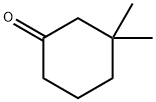
3,3-Dimethylcyclohexanone synthesis
- Product Name:3,3-Dimethylcyclohexanone
- CAS Number:2979-19-3
- Molecular formula:C8H14O
- Molecular Weight:126.2
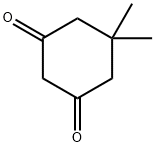
126-81-8
418 suppliers
$5.00/10g

2979-19-3
238 suppliers
$6.00/1g
Yield:2979-19-3 98%
Reaction Conditions:
with hydrogen;Amberlyst CH57 in methanol at 85; under 3750.38 Torr; for 7.5 h;Product distribution / selectivity;Autoclave;
Steps:
3
General procedure for examples 1-7:; Amberlyst CH57 (1 g cat per g dimedone; = 1 mol% Pd in relation to dimedone) was placed in a glass-liner. 4.38 g (31 mmol) of dimedone and ca. 15 g of the solvent (leading to a 20 weight-% solution of dimedone) were added. The glass-liner was closed and stirring was started. The autoclave was flushed three times with 5 bara N2. The stirrer was turned off. The autoclave was pressurized with 5 bara H2 (i.e. 1 bar atmospheric pressure + 4 bar H2 pressure) for 10 minutes for pressure check. The pressure was released. The stirrer was turned on again and the autoclave was heated to 85°C internal temperature. The autoclave was pressurized with H2 and the stirrer was set on. The reaction mixture was stirred under H2-pressure at 85°C until hydrogen uptake had ceded (when the hydrogenation curve is horizontal, usually after 110% of theoretical H2-amount). Then the autoclave was cooled to a temperature below 25°C and depressurized. After three times flushing with N2 at 5 bara for 10 minutes stirring was stopped and the autoclave was opened. The content was sucked and filtrated over a 0.45 μm filter. A 30 μL filtrated sample was diluted with 1 mL of isopropanol and 5 mg of NaHCO3 and then analyzed by GC.; * Example 3: Exp. 20804 Hydrogenation in methanol at a larger scale; 37 g of Amberlyst CH57 were placed in a 500 mL glass-liner. 32 g of dimedone (221 mmol) and 116 g of methanol (147 mL) were added. The glass-liner was closed and stirring was started with 500 rpm. The autoclave was flushed three times with 5 bara N2. The stirrer was turned off. The autoclave was pressurized with 5 bara H2 for 10 minutes for pressure check. The pressure was released. The stirrer was turned on to 1000 rpm and the autoclave was heated to 85°C internal temperature. The autoclave was pressurized with 2 bara H2 and the stirrer was set to 1000 rpm. The reaction mixture was stirred under 2 bara H2 at 85°C for 7.5 hours. Then the autoclave was cooled to a temperature below 25°C and depressurized. After three times flushing with N2 at 5 bara for 10 minutes stirring was stopped and the autoclave was opened. The content was sucked and filtrated over a 0.45 μm filter. A 30 μL filtrated sample was diluted with 1 mL of isopropanol and 5 mg of NaHCO3 and then analyzed by GC. The total yield of 3,3-dimethylcyclohexanone was 98% with a selectivity of 98 %. Here 37 g of Amberlyst CH57; 32 g of dimedone (221 mmol) and 116 g of methanol (147 mL) were used showing that the process of the present invention may also be successfully, i.e. with high yield and selectivity, carried out at a larger scale.
References:
EP2128118,2009,A1 Location in patent:Page/Page column 7-8
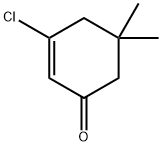
17530-69-7
76 suppliers
$47.29/5g

2979-19-3
238 suppliers
$6.00/1g
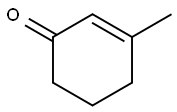
1193-18-6
174 suppliers
$6.00/1g

2979-19-3
238 suppliers
$6.00/1g
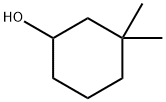
767-12-4
17 suppliers
inquiry

2979-19-3
238 suppliers
$6.00/1g