
2-(TETRADECYLOXY)ETHANOL synthesis
- Product Name:2-(TETRADECYLOXY)ETHANOL
- CAS Number:2136-70-1
- Molecular formula:C16H34O2
- Molecular Weight:258.44
Yield:2136-70-1 65%
Reaction Conditions:
Stage #1:ethylene glycol with sodium hydride in N,N-dimethyl-formamide at 0; for 0.166667 h;
Stage #2:1-Bromotetradecane with potassium iodide in N,N-dimethyl-formamide at 95; for 4 h;
Steps:
1 Synthesis of 0170
Sodium hydride (0.72 g, 30 mmol) was added to the solution of ethylene glycol (5.6 g, 90 mmol) in anhydrous DMF (30 mL) and stirred for 10 min at 0 °C. 1- Bromotetradecane (6.0 g, 20 mmol) and KI (3.3 g, 20 mmol) were then added and the reaction mixture was kept at 95 °C for another 4 h. After cooling to room (0210) temperature, the mixture was diluted with cold water, extracted with ethyl acetate, and dried over anhydrous sodium sulfate. Compound 1 (3.3 g, yield about 65%) was obtained after column chromatography purification on silica gel using n-hexane/ethyl acetate as mobile phase. Then, compound 1 (3.3 g, 12.8 mmol) and triethylamine (TEA, 1.9 g, 19.2 mmol) were dissolved in anhydrous DCM (80 mL). Acryloyl chloride (1.4 g, 15.4 mmol) was added drop wise at 0 °C, and the reaction mixture was stirred overnight. After column chromatography purification, 0170 was obtained as colorless oil (3.2 g, yield about 82%). The structure of 0170 was confirmed by 1 H NMR spectrum recorded in CDCI3
References:
TRUSTEES OF TUFTS COLLEGE;XU, Qiaobing;LI, Yamin WO2019/152848, 2019, A1 Location in patent:Page/Page column 31; 32

112-72-1
370 suppliers
$5.00/25g

2136-70-1
15 suppliers
inquiry
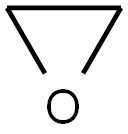
75-21-8
233 suppliers
$33.29/crm44609

112-72-1
370 suppliers
$5.00/25g

2136-70-1
15 suppliers
inquiry

112-53-8
449 suppliers
$10.00/5g

112-72-1
370 suppliers
$5.00/25g

4536-30-5
55 suppliers
$186.00/1ml

3055-93-4
67 suppliers
$431.00/5g

56049-79-7
7 suppliers
inquiry

2136-70-1
15 suppliers
inquiry

