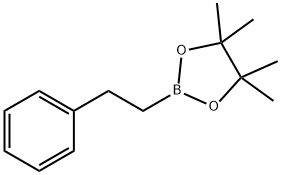
2-PHENYLETHYL-1-BORONIC ACID PINACOL ESTER synthesis
- Product Name:2-PHENYLETHYL-1-BORONIC ACID PINACOL ESTER
- CAS Number:165904-22-3
- Molecular formula:C14H21BO2
- Molecular Weight:232.13
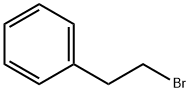
103-63-9
513 suppliers
$9.00/10g
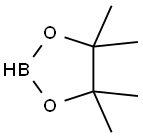
25015-63-8
224 suppliers
$22.39/5G

165904-22-3
50 suppliers
$21.00/1g
Yield: 80%
Reaction Conditions:
Stage #1:4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane with bis(cyclopentadienyl)titanium dichloride;lithium methanolate in tert-butyl methyl ether for 0.5 h;
Stage #2:1-phenyl-2-bromoethane in tert-butyl methyl ether at 60; under 760.051 Torr; for 24 h;Inert atmosphere;
Steps:
52 Example 52
bis(cyclopentadiene) titanium dichloride (denoted as Cp2TiCl2, 0.01 mmol, 2.5 mg), lithium methoxide (denoted as MeOLi, 0.2 mmol, 7.6 mg), methyl tert-butyl ether (1 mL) Hopinacol borane (denoted as HBpin, 0.4mmol,58μL) was added to the 38mL pressure tube in sequence, stirred for 30min, and then 1-bromophenylethane (denoted as 1m, 0.2mmol, 27.3μL) was added in nitrogen (1atm ) Stir at 60°C for 24h under an atmosphere, and purify by column chromatography with petroleum ether/ethyl acetate (40:1, v/v) as the eluent to obtain the structure compound represented by formula 2m (colorless transparent liquid, 2-(2) -Phenylethyl) borate pinacol ester). The isolated yield is 80%.
References:
CN112645971, 2021, A Location in patent:Paragraph 0145-0147

103-63-9
513 suppliers
$9.00/10g
73183-34-3
593 suppliers
$6.00/5g

165904-22-3
50 suppliers
$21.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![1,3,2-Dioxaborolane, 2-[(1S)-1-chloro-2-phenylethyl]-4,4,5,5-tetramethyl-](/CAS/20210305/GIF/1259299-92-7.gif)
1259299-92-7
0 suppliers
inquiry

165904-22-3
50 suppliers
$21.00/1g
622-24-2
273 suppliers
$5.00/5g

73183-34-3
593 suppliers
$6.00/5g

165904-22-3
50 suppliers
$21.00/1g

622-24-2
273 suppliers
$5.00/5g

25015-63-8
224 suppliers
$22.39/5G

165904-22-3
50 suppliers
$21.00/1g