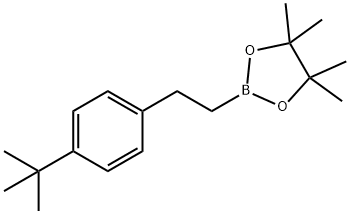
2-(4-tert-Butylphenyl)ethylboronic acid pinacol ester synthesis
- Product Name:2-(4-tert-Butylphenyl)ethylboronic acid pinacol ester
- CAS Number:1073355-22-2
- Molecular formula:C18H29BO2
- Molecular Weight:288.23
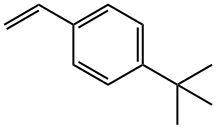
1746-23-2
119 suppliers
$9.00/25g
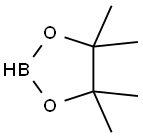
25015-63-8
221 suppliers
$22.39/5G

1073355-22-2
11 suppliers
$51.39/1g
Yield:1073355-22-2 99%
Reaction Conditions:
with calcium(II) chloride in chloroform-d1 at 110; for 12 h;Glovebox;
Steps:
12 Calcium chloride catalyzes the reaction of 4-tert-butylstyrene with pinacol borane, the process is as follows:
In the glove box, add calcium chloride 3 mol%, 4-tert-butylstyrene 0.2 mmol, pinacol borane 0.4 mmol, CDCl3 50 μl into the reaction flask in sequence, then remove it from the glove box, stir at 110 ° C for 12 h, 57.0 mg of product were obtained in 99% yield by NMR spectroscopy.
References:
CN113880873,2022,A Location in patent:Paragraph 0090-0095
76-09-5
384 suppliers
$10.00/1g

1746-23-2
119 suppliers
$9.00/25g
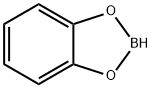
274-07-7
172 suppliers
$78.09/5g

1073355-22-2
11 suppliers
$51.39/1g

1746-23-2
119 suppliers
$9.00/25g
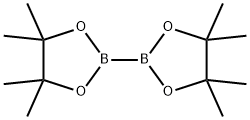
73183-34-3
581 suppliers
$6.00/5g

1073355-22-2
11 suppliers
$51.39/1g
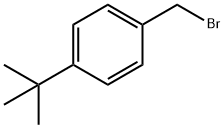
18880-00-7
213 suppliers
$9.00/5g
![Bis[(pinacolato)boryl]Methane](/CAS/GIF/78782-17-9.gif)
78782-17-9
98 suppliers
$22.00/1g

1073355-22-2
11 suppliers
$51.39/1g
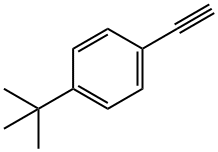
772-38-3
119 suppliers
$31.92/2g

73183-34-3
581 suppliers
$6.00/5g

1073355-22-2
11 suppliers
$51.39/1g