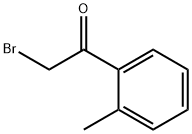
2'-METHYLPHENACYL BROMIDE synthesis
- Product Name:2'-METHYLPHENACYL BROMIDE
- CAS Number:51012-65-8
- Molecular formula:C9H9BrO
- Molecular Weight:213.07
933-88-0
354 suppliers
$10.00/1g
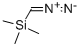
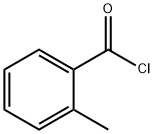
18107-18-1
214 suppliers
$33.00/5g

51012-65-8
118 suppliers
$26.00/200mg
Yield: 91%
Reaction Conditions:
with hydrogen bromide in hexane;water;ethyl acetate;acetonitrile
Steps:
6 Preparation of 2-bromo-1-o-tolylethanone (I15)
Example 6 Preparation of 2-bromo-1-o-tolylethanone (I15) To a solution of 2-methylbenzoyl chloride (169 μl, 1.29 mmol) in dry acetonitrile (5 ml) and cooled at 0° C., under nitrogen atmosphere, was added (diazomethyl)-trimethylsilane (1.94 ml, 3.88 mmol, 2M in hexane). The reaction was stirred at room temperature for 15 hours, then it was cooled at 0° C. and 48% HBr (512 μl, 4.53 mmol) was slowly added. The reaction was stirred at room temperature for 3 hours. EtOAc and water were added, the organic layer was separated and the aqueous phase was neutralized with 1M NaOH and extracted with EtOAc. The combined organic layers were dried over Na2SO4, filtered and evaporated to dryness to obtain 2-bromo-1-o-tolylethanone (250 mg, 91% yield). This intermediate was used in the next step without any further purification. 1H NMR (300 MHz, DMSO-d6) δ ppm 7.82-7.91 (m, 1H), 7.45-7.52 (m, 1H), 7.25-7.40 (m, 2H), 4.86 (s, 2H), 2.41 (s, 3H).
References:
Chiesi Farmaceutici S.p.A. US2011/311458, 2011, A1
611-15-4
123 suppliers
$128.00/5g

51012-65-8
118 suppliers
$26.00/200mg

933-88-0
354 suppliers
$10.00/1g

51012-65-8
118 suppliers
$26.00/200mg

75-15-0
262 suppliers
$40.00/100 g

7446-70-0
713 suppliers
$15.00/25g
106-38-7
415 suppliers
$14.00/5g
75-36-5
589 suppliers
$17.92/100G

51012-65-8
118 suppliers
$26.00/200mg

75-15-0
262 suppliers
$40.00/100 g

7446-70-0
713 suppliers
$15.00/25g

95-46-5
308 suppliers
$10.00/10 g

75-36-5
589 suppliers
$17.92/100G

51012-65-8
118 suppliers
$26.00/200mg