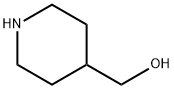
4-Piperidinemethanol synthesis
- Product Name:4-Piperidinemethanol
- CAS Number:6457-49-4
- Molecular formula:C6H13NO
- Molecular Weight:115.17
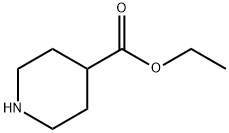
1126-09-6
490 suppliers
$15.40/25g

6457-49-4
388 suppliers
$18.00/25g
Yield:6457-49-4 100%
Reaction Conditions:
Stage #1: 4-carbethoxypiperidinewith lithium aluminium tetrahydride in tetrahydrofuran at 20;
Stage #2: with sodium hydroxide;water in tetrahydrofuran at 20; for 0.5 h;
Steps:
28.a; 29.a EXAMPLE 28; 2-[1-(tert-Butoxycarbonyl)piperidin-4-ylmethyl]-4, 6-bis (4-FLUOROPHENYL)-5-(4- PYRIDYL) PYRAZOLO [3, 4-B] PYRIDINE; EXAMPLE 29; 1- [1- (TERT BUTOXYCARBONYL) PIPERIDIN-4-YLMETHYL]-4, 6-BIS (4-FLUOROPHENYL)-5- (4- PYRIDYL) PYRAZOLOL3, 4-B] PYRIDINE; A) (4-PIPERIDYL) METHANOL
To A suspension of LIAIH4 (10. 10 G, 0. 266 MOL) IN THF (150 ML) AT 0 C, A SOLUTION OF ETHYL PIPERIDINE-4-CARBOXYLATE (18. 13 G, 0. 120 MOL) IN THF (300 ML) WAS ADDED slowly. This was stirred at room temperature overnight. It was cooled with an ice bath and A mixture of water (14 ML) and THF (28 ML) was added slowly. Afterwards, A mixture of 15% aqueous NAOH (14 mL) and water (37 ML) was added followed by stirring at room temperature for 30 min. The precipitate obtained was filtered and the filtrate was concentrated, to afford 17. 88 g of the desired compound (yield : quantitative).
References:
WO2004/76450,2004,A1 Location in patent:Page 59
498-94-2
0 suppliers
$5.00/10g

6457-49-4
388 suppliers
$18.00/25g
586-95-8
377 suppliers
$6.00/1g

6457-49-4
388 suppliers
$18.00/25g
![2H-Pyran-4-carboxylic acid, tetrahydro-4-[(4-hydroxy-1-piperidinyl)sulfonyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/622387-50-2.gif)
622387-50-2
0 suppliers
inquiry
4282-40-0
183 suppliers
$19.00/10g

6457-49-4
388 suppliers
$18.00/25g
123855-51-6
426 suppliers
$6.00/5g

6457-49-4
388 suppliers
$18.00/25g