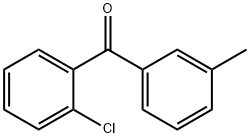
2-CHLORO-3'-METHYLBENZOPHENONE synthesis
- Product Name:2-CHLORO-3'-METHYLBENZOPHENONE
- CAS Number:131822-46-3
- Molecular formula:C14H11ClO
- Molecular Weight:230.69

108-88-3
7 suppliers
$14.00/100ml
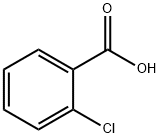
118-91-2
647 suppliers
$14.00/25g
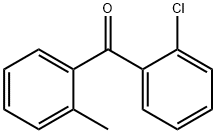
4888-03-3
9 suppliers
inquiry

131822-46-3
12 suppliers
$294.00/1g
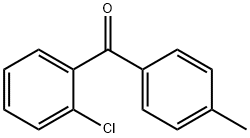
5953-00-4
15 suppliers
$45.00/5mg
Yield:-
Reaction Conditions:
with erbium(III) triflate in neat (no solvent) at 180; for 0.333333 h;Microwave irradiation;Overall yield = 85 %;Friedel-Crafts Acylation;
Steps:
General procedure
General procedure: mixture of Er(OTf)3 (0.0614 g, 0.1 mmol), anisole(0.5407 g, 5 mmol) and benzoic acid (0.1221 g, 1 mmol) was heated undermicrowave irradiation at 220 C for 30 min in a CEM Discover apparatus. Afterbeing cooled, the mixture was extracted with CH2Cl2 (3 15 mL). The organiclayer was decanted, washed with H2O (10 mL), aqueous NaHCO3 (2 20 mL),and brine (10 mL), and dried over MgSO4. The solvent was removed on a rotaryevaporator. The crude product was purified by flash chromatography (nhexane,then 10% EtOAc in n-hexane) to give 4-methoxybenzophenone(0.153 g, 72% yield). The purity and identity of the product were confirmedby GC-FID, and from GC-MS spectra which were compared with the spectra inthe NIST library, and by 1H and 13C NMR spectroscopy.
References:
Tran, Phuong Hoang;Hansen, Poul Erik;Nguyen, Hai Truong;Le, Thach Ngoc [Tetrahedron Letters,2015,vol. 56,# 4,p. 612 - 618]
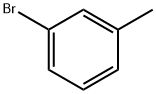
591-17-3
252 suppliers
$10.00/10g
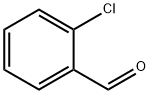
89-98-5
640 suppliers
$15.00/25g

131822-46-3
12 suppliers
$294.00/1g

591-17-3
252 suppliers
$10.00/10g

131822-46-3
12 suppliers
$294.00/1g

89-98-5
640 suppliers
$15.00/25g

131822-46-3
12 suppliers
$294.00/1g