
2,6-Dichloronitrobenzene synthesis
- Product Name:2,6-Dichloronitrobenzene
- CAS Number:601-88-7
- Molecular formula:C6H3Cl2NO2
- Molecular Weight:192
608-31-1
316 suppliers
$12.00/25g

601-88-7
166 suppliers
$8.00/5g
Yield:601-88-7 92%
Reaction Conditions:
Stage #1:2,6-Dichloroaniline with sodium tungstate (VI) dihydrate;sulfuric acid in methanol at 40;
Stage #2: with dihydrogen peroxide in methanol;water; pH=0.5 at 40; for 9 h;
Stage #3: with potassium hydroxide in methanol;water at 20 - 40; for 2 h;Reagent/catalyst;pH-value;Temperature;Solvent;
Steps:
1 Example 1 Production of 2,6-Dichloronitrobenzene
1.32 g (4.0 mmol) of sodium tungstate dihydrate and 4.0 g (40 mmol) of concentrated sulfuric acid were added to a solution of 16.2 g (100 mmol) of 2,6-dichloroaniline in 120 ml of methanol, and the mixture was heated to 40° C. 30 ml (291 mmol) of a 30% hydrogen peroxide solution was added dropwise over 10 hours.
The pH value at this time was 0.5.
After the completion of the dropwise addition, the mixture was stirred at 40° C. for 9 hours.
After the disappearance of the 2,6-dichloroaniline was confirmed by gas chromatography (area percentage method), a solution of 9.8 g (150 mmol) of 86% potassium hydroxide in 24.3 ml of methanol was added dropwise while the temperature of the reaction liquid was adjusted to 40° C. or less.
After the completion of the dropwise addition, the mixture was stirred at room temperature for 2 hours, and the reaction was completed.
After the completion of the reaction, 95 ml of toluene and 32 ml of water were added, and the mixture was stirred for a while, and filtered.
The filtrate was separated.
The obtained organic phase was washed with 8 ml of water, and then heated to reflux, and dehydrated.
Thus, 2,6-dichloronitrobenzene was obtained as a toluene solution.
This solution was analyzed by gas chromatography by the absolute calibration method, and as a result, the yield was 92%.
References:
IHARA CHEMICAL INDUSTRY CO., LTD.;Tani, Shinki;Yadomatsu, Nami;Ikumi, Akiko;Hirano, Yuuki;Ido, Takuya US2014/163256, 2014, A1 Location in patent:Paragraph 0161-0162
5942-05-2
5 suppliers
inquiry

601-88-7
166 suppliers
$8.00/5g

15307-79-6
894 suppliers
$14.00/5g
59-48-3
479 suppliers
$5.00/1g
87-65-0
415 suppliers
$10.00/10 g
15362-40-0
337 suppliers
$8.00/5g
69220-40-2
19 suppliers
inquiry

601-88-7
166 suppliers
$8.00/5g
15307-93-4
238 suppliers
$7.00/5g
10113-35-6
26 suppliers
inquiry
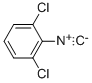
6697-95-6
14 suppliers
$45.00/10mg
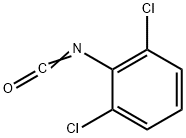
39920-37-1
95 suppliers
$15.00/1g
![4-chlorobenzo[d]oxazol-2(3H)-one](/CAS/20180702/GIF/13603-93-5.gif)
13603-93-5
6 suppliers
inquiry

608-31-1
316 suppliers
$12.00/25g
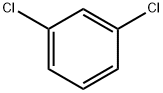
541-73-1
412 suppliers
$19.00/25G
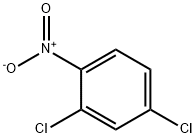
611-06-3
24 suppliers
$15.00/25g
618-62-2
172 suppliers
$47.00/25g

601-88-7
166 suppliers
$8.00/5g