
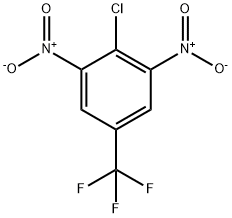
2,4-Dichloro-3,5-dinitrobenzotrifluoride synthesis
- Product Name:2,4-Dichloro-3,5-dinitrobenzotrifluoride
- CAS Number:29091-09-6
- Molecular formula:C7HCl2F3N2O4
- Molecular Weight:304.99
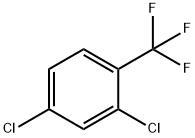
320-60-5
295 suppliers
$5.00/10g

29091-09-6
239 suppliers
$16.00/1g
Yield:29091-09-6 166.6 grams (79%)
Reaction Conditions:
with sulfuric acid;sulfur trioxide;nitric acid;sodium hydrogencarbonate in water;toluene
Steps:
IV 2,4-Dichloro-3,5-dinitrobenzotrifluoride
EXAMPLE IV 2,4-Dichloro-3,5-dinitrobenzotrifluoride Fuming sulfuric acid (600 ml.) containing 30-33 percent free SO3 was stirred in a two-liter, three-necked flask immersed in an ice bath. Fuming 90 percent nitric acid (585 ml.) was added followed by 148.8 grams (0.692 mole) of 2,4-dichlorobenzotrifluoride. This stirred slurry then was heated to 76° C. and held at 76°+-1° C. for 96 hours. The mixture was cooled and the acid was drained from the crust of crystalline product. Water (1,000 ml.) was added to the broken up solid and the stirred slurry extracted with 500 ml. of toluene. The toluene solution, with another 500 ml. of toluene added, was washed successively with water (500 ml.), twice with 500 ml. of 5 percent sodium bicarbonate solution, and finally with water (500 ml.). Removal of the toluene by evaporation at reduced pressure and drying overnight gave 166.6 grams (79%) of the desired 2,4-dichloro-3,5-dinitrobenzotrifluoride, m.p. 67°-72°
References:
United States Borax & Chemical Corp. US4201724, 1980, A
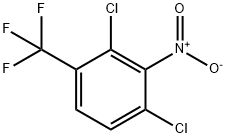
203915-49-5
30 suppliers
$47.00/1g

29091-09-6
239 suppliers
$16.00/1g
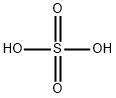
7664-93-9
0 suppliers
$21.64/1003
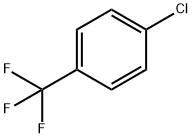
98-56-6
401 suppliers
$11.00/25g
393-75-9
290 suppliers
$10.00/5g

29091-09-6
239 suppliers
$16.00/1g