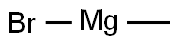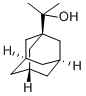
2-(1-Adamantyl)propan-2-ol synthesis
- Product Name:2-(1-Adamantyl)propan-2-ol
- CAS Number:775-64-4
- Molecular formula:C13H22O
- Molecular Weight:194.31
Yield:775-64-4 85.5%
Reaction Conditions:
Stage #1:methyl iodide with magnesium in diethyl ether
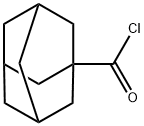
Stage #2:1-Adamantanecarbonyl chloride in diethyl ether for 4 h;Inert atmosphere;Reflux;
Steps:
[00135] The Grignard reagent was prepared from magnesium turnings (1 .99 g, 83.1 mmol) and methyl iodide (10.7 g, 75.6mmol) in 40ml_ of dry diethyl ether. A solution of 1 -adamantanecarbonyl chloride 202 (2.5 g, 12.6mmol) in 60ml_ of dry diethyl ether was added dropwise under Ar atmosphere and stirring. The reaction mixture was heated at gentle reflux for 4 hours under stirring and Ar atmosphere. The mixture was treated with an equal volume of saturated solution of ammonium chloride under ice-cooling. The organic layer was separated and the aqueous phase was extracted with diethyl ether 2 times. The combined organic phases were washed with water and brine, dried (Na2S04) and evaporated under vacuum to yield a white colored solid residue of 2-(1 -adamantyl)-propan-2-ol 203. Yield 2.09 g (85.5%); IR (Nujol): v(OH) 3400 (br.s, O-H) cm"1 ; 1 H-NMR (400MHz, CDCI3) δ: 1 .12 (s, 6H), 1 .63 (d, 9H), 1 .67 (d, 3H), 1 .99 (br s, 3H); 13C-NMR (200MHz, CDCI3) δ: 24.34 (CH3), 28.74 36.35 (C4.,6.,i0 Ad), 37.22 38.84 (C Ad), 74.88 (C-OH).
References:
BRIGHAM YOUNG UNIVERSITY;KOLOCOURIS, Antonios;BUSATH, David, D.;JOHNSON, Brent WO2014/121170, 2014, A2 Location in patent:Paragraph 00135
711-01-3
170 suppliers
$40.00/2 g
917-54-4
180 suppliers
$44.39/50ml

74-88-4
352 suppliers
$15.00/10g

775-64-4
77 suppliers
inquiry