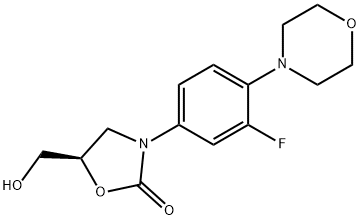
(5R)-3-(3-Fluoro-4-(4-morpholinyl)phenyl)-5-hydroxymethyl-2-oxazolidione synthesis
- Product Name:(5R)-3-(3-Fluoro-4-(4-morpholinyl)phenyl)-5-hydroxymethyl-2-oxazolidione
- CAS Number:168828-82-8
- Molecular formula:C14H17FN2O4
- Molecular Weight:296.29
![Carbamic acid, [3-fluoro-4-(4-morpholinyl)phenyl]-, ethyl ester](/CAS/20200331/GIF/565176-83-2.gif)
565176-83-2
7 suppliers
inquiry
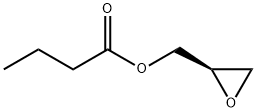
60456-26-0
311 suppliers
$14.14/1gm:

168828-82-8
253 suppliers
$5.00/250mg
Yield: 80%
Reaction Conditions:
Stage #1:N-ethoxycarbonyl-3-fluoro-4-morpholinyl aniline with n-butyllithium in tetrahydrofuran;hexane at -78; for 3.5 h;Inert atmosphere;
Stage #2:(R)-glycidyl butyrate in tetrahydrofuran;hexane at -78 - 20;
Stage #3: with ammonium chloride in tetrahydrofuran;hexane;water at 20; for 0.5 h;Product distribution / selectivity;
Steps:
5.a
Example-S(a): Preparation of (R)-[N-3-(3-fluoro-4-morpholinylphenyl)-2- oxo-5- oxazolidinyljmethanol (VIII) starting from N-carboethoxy-3-fluoro-4- morpholinylanilineN-carboethoxy-3-fluoro-4-morpholinylaniline (100 g) was dissolved in THF (500 ml). To the resulting solution, n-butyllithium (245 ml in hexane) was added under nitrogen at -78°C over 1.5h and stirred for 2h, further a solution of R-(-)-glycidyl butyrate (53.75 g, in THF (100 ml)) was added. The resulting solution was stirred at -78°C for 2h, further it was warmed to room temperature and stirred for overnight. To the resulting thick slurry, saturated ammonium chloride (345 ml) was added followed by the addition of water (300 ml). It was stirred at room temperature for 30 min and was concentrated. Further, water (500 ml) was added and the suspension was again stirred at 50-55°C for lh and then at 25-30°C for next lh. The solid mass was filtered, washed with water and suck dried for lh. The filtrate was extracted with toluene. To the combined toluene layer, the suck dried material obtained above was added and heated to reflux and further concentrated. The concentrated solution was cooled to room temperature and stirred for lh. The solid mass was filtered, washed with toluene and dried to obtain the titled compound (110 g) with 80% yield.
References:
JUBILANT LIFE SCIENCES LIMITED;GUPTA, Ashish, Kumar;SINGH, Shishupal;PANDA, Atulya, Kumar;BISWAS, Sujay;VIR, Dharam WO2011/114210, 2011, A2 Location in patent:Page/Page column 20
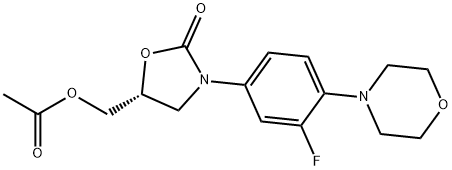
496031-56-2
24 suppliers
inquiry

168828-82-8
253 suppliers
$5.00/250mg
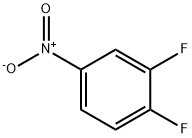
369-34-6
416 suppliers
$5.00/5g

168828-82-8
253 suppliers
$5.00/250mg
2689-39-6
159 suppliers
$10.00/1g

168828-82-8
253 suppliers
$5.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

168828-82-8
253 suppliers
$5.00/250mg