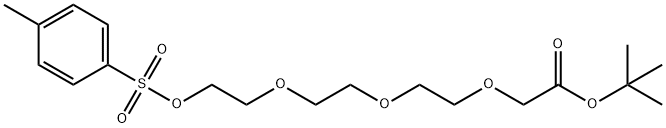
(2-{2-[2-(Toluene-4-sulfonyloxy)-ethoxy]-ethoxy}-ethoxy)-acetic acid tert-butyl ester synthesis
- Product Name:(2-{2-[2-(Toluene-4-sulfonyloxy)-ethoxy]-ethoxy}-ethoxy)-acetic acid tert-butyl ester
- CAS Number:1530778-24-5
- Molecular formula:C19H30O8S
- Molecular Weight:418.5
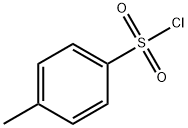
98-59-9
616 suppliers
$9.00/5g
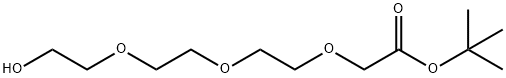
518044-31-0
85 suppliers
$92.00/1 g
![(2-{2-[2-(Toluene-4-sulfonyloxy)-ethoxy]-ethoxy}-ethoxy)-acetic acid tert-butyl ester](/CAS/GIF/1530778-24-5.gif)
1530778-24-5
45 suppliers
$28.00/100mg
Yield: 82.1%
Reaction Conditions:
with pyridine at 20; for 16 h;
Steps:
10.10 Step 10: Synthesis of tert-butyl 2-(2-(2-(2-(tosyloxy)ethoxy)ethoxy)ethoxy)acetate (17)
To a mixture of tert-butyl 2-[2-[2-(2-hydroxyethoxy)ethoxy]ethoxy]acetate (1 g, 3.78 mmol) in pyridine (10 mL), 4-methylbenzenesulfonyl chloride (1.08 g, 5.68 mmol) was added, The mixture was stirred at 20 °C for 16 hours. According to the LCM, the main peak of the desired mass appeared. TLC (petroleum ether/ethyl acetate=1:1) showed a main spot was formed. The mixture was poured into water (20 mL) and extracted with ethyl acetate (30 mL). The organic layer was washed with 1 N HCl aqueous solution (20 mL), It was dried over anhydrous Na2SO4 and concentrated. The residue was purified by chromatography (silica gel, eluting with petroleum ether/ethyl acetate=5:1 to 1:1) to give the title compound (1.3 g, 3.11 mmol, 82.10% yield) as a colorless oil.
References:
Eop Te Ra Co., Ltd.;Ryu Su-hui;Lee Hwa-jin;Kim Seong-hun;Ryu Hye-guk;Lee Eun-bin KR102160377, 2020, B1 Location in patent:Paragraph 0461; 0496-0499
5292-43-3
456 suppliers
$10.00/5g
![Ethanol, 2-[2-(2-hydroxyethoxy)ethoxy]-, 1-(4-Methylbenzenesulfonate)](/CAS/GIF/77544-68-4.gif)
77544-68-4
97 suppliers
$49.00/100mg
![(2-{2-[2-(Toluene-4-sulfonyloxy)-ethoxy]-ethoxy}-ethoxy)-acetic acid tert-butyl ester](/CAS/GIF/1530778-24-5.gif)
1530778-24-5
45 suppliers
$28.00/100mg

5292-43-3
456 suppliers
$10.00/5g
![(2-{2-[2-(Toluene-4-sulfonyloxy)-ethoxy]-ethoxy}-ethoxy)-acetic acid tert-butyl ester](/CAS/GIF/1530778-24-5.gif)
1530778-24-5
45 suppliers
$28.00/100mg
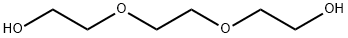
112-27-6
772 suppliers
$6.00/100g
![(2-{2-[2-(Toluene-4-sulfonyloxy)-ethoxy]-ethoxy}-ethoxy)-acetic acid tert-butyl ester](/CAS/GIF/1530778-24-5.gif)
1530778-24-5
45 suppliers
$28.00/100mg
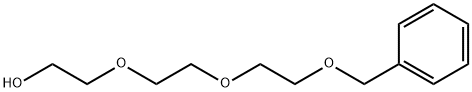
55489-58-2
127 suppliers
$12.00/1g
![(2-{2-[2-(Toluene-4-sulfonyloxy)-ethoxy]-ethoxy}-ethoxy)-acetic acid tert-butyl ester](/CAS/GIF/1530778-24-5.gif)
1530778-24-5
45 suppliers
$28.00/100mg