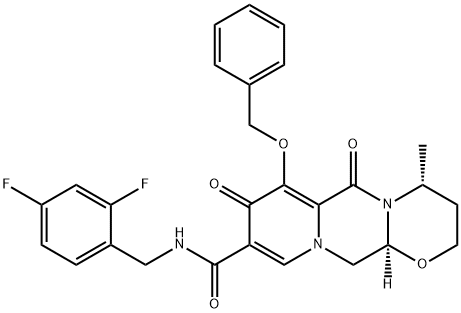
(4R,12aS)-N-(2,4-difluorobenzyl)-7-benzylhydroxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxaMide synthesis
- Product Name:(4R,12aS)-N-(2,4-difluorobenzyl)-7-benzylhydroxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxaMide
- CAS Number:1206102-11-5
- Molecular formula:C27H25F2N3O5
- Molecular Weight:509.5
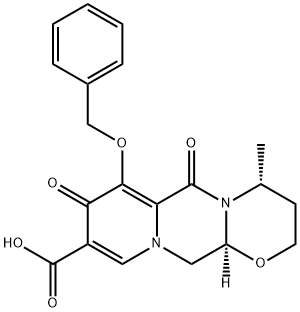
1339879-91-2
7 suppliers
inquiry
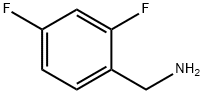
72235-52-0
405 suppliers
$12.00/5g
![(4R,12aS)-N-(2,4-difluorobenzyl)-7-benzylhydroxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxaMide](/CAS/20150408/GIF/1206102-11-5.gif)
1206102-11-5
87 suppliers
inquiry
Yield: 93.3%
Reaction Conditions:
with 1,1'-carbonyldiimidazole in dichloromethane for 36 h;
Steps:
Preparation of (4R,12aS)-N-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-4-methyl-6,8-dioxo-7-(phenylmethoxy)-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide (DTG-1):
A 3 L round bottom flask was charged with 180.0 g of (4R, 12aS)- 3 ,4,6,8, 12, 12a-hexahydro-4-methyl-6, 8-dioxo-7-(phenylmethoxy)-2H- Pyrido[l',2':4,5]pyrazino[2,l-b][l ,3]oxazine-9-carboxylic acid (DTG-2) (0.47 mol, 1.00 eq.) and 1.0 L dichloromethane. To this solution, at room temperature, a slurry of 98.6 g N.N'-carbonyldiimidazole (0.61 mol, 1.30 eq.) in 500 ml dichloromethane was added in portions. The mixture was stirred at RT until complete consumption of starting material (approx. 7 h). Then 2,4 difluorobenzyl amine was added dropwise three times, i.e. initially 67.0 g (0.47 mol, 1.00 eq.) in 160 ml dichloromethane, secondly after 15 h stirring at RT another portion of 13.4 g (93.6 mmol, 0.20 eq.) and, finally, after 6 h stirring, 3.80 g 2,4 difluorobenzyl amine (26.6 mmol, 0.05 eq.). After the third portion, the mixture was stirred for another 15 h at RT. The reaction mixture was washed with 10% HC1 solution (620 ml), 10% sodium carbonate (400 ml), water (3 x 675 ml). The organic phase was dried and concentrated under reduced pressure. The residue was crystallized in 800 ml isopropanol, collected by filtration, washed with isopropanol (400 ml) and dried at room temperature under vacuum to give 222.5 g of 4R,12aS)-N-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a- hexahydro-4-methyl-6,8-dioxo-7-(phenylmethoxy)-2H-Pyrido[l ',2':4,5]pyrazino[2,l- b][l,3]oxazine-9-carboxamide (93.3% yield) as off-white solid. Chemical purity: 96.6% (HPLC, peak area at λ=254 nm). 1H NMR (400 MHz, CDCl3, 5 ppm): 1.32 (d, 3H, J = 7.0 Hz), 1.49 (dd, 1H, J = 13.9, 1.8 Hz), 2.0 - 2.2 (m, 1H), 3.8 - 4.0 (m, 2H), 4.09 (dd, 1H, J = 13.5, 5.7 Hz), 4.22 (dd, 1H, J = 13.3, 3.5 Hz), 4.32 (s, 1H), 4.63 (d, 2H, J = 5.9 Hz), 4.9 - 5.1 (m, 1H), 5.13 (dd, 1H, J = 5.5, 3.9 Hz), 5.2 - 5.3 (m, 2H), 6.7 - 6.9 (m, 3H), 7.2 - 7.4 (m, 4H), 7.61 (d, 2H, J = 6.7 Hz), 8.36 (br. s., 1H), 10.40 (br. s., 1H)
References:
RATIOPHARM GMBH;TEVA PHARMACEUTICALS USA, INC.;ALBRECHT, Wolfgang;COUTABLE, Ludovic;KOELLNER, Gertraud WO2015/9927, 2015, A1 Location in patent:Page/Page column 23; 24
![Methyl 5-[[[(2,4-difluorophenyl)methyl]amino]carbonyl]-1-(2-oxoethyl)-4-oxo-3-[(phenylmethyl)oxy]-1,4-dihydro-2-pyridinecarboxylate](/CAS/20150408/GIF/1229006-25-0.gif)
1229006-25-0
17 suppliers
inquiry
61477-40-5
443 suppliers
$15.00/1g
![(4R,12aS)-N-(2,4-difluorobenzyl)-7-benzylhydroxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxaMide](/CAS/20150408/GIF/1206102-11-5.gif)
1206102-11-5
87 suppliers
inquiry
201230-82-2
1 suppliers
inquiry
![(4R,12aS)-7-(benzyloxy)-9-broMo-4-Methyl-3,4-dihydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-6,8(12H,12aH)-dione](/CAS/20150408/GIF/1206102-10-4.gif)
1206102-10-4
24 suppliers
$1131.00/1g

72235-52-0
405 suppliers
$12.00/5g
![(4R,12aS)-N-(2,4-difluorobenzyl)-7-benzylhydroxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxaMide](/CAS/20150408/GIF/1206102-11-5.gif)
1206102-11-5
87 suppliers
inquiry
![(S)-7-phenylMethoxy-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxylic acid Methyl ester](/CAS/20150408/GIF/1357289-10-1.gif)
1357289-10-1
2 suppliers
inquiry

72235-52-0
405 suppliers
$12.00/5g
![(4R,12aS)-N-(2,4-difluorobenzyl)-7-benzylhydroxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxaMide](/CAS/20150408/GIF/1206102-11-5.gif)
1206102-11-5
87 suppliers
inquiry
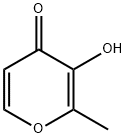
118-71-8
618 suppliers
$5.00/25mg
![(4R,12aS)-N-(2,4-difluorobenzyl)-7-benzylhydroxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxaMide](/CAS/20150408/GIF/1206102-11-5.gif)
1206102-11-5
87 suppliers
inquiry