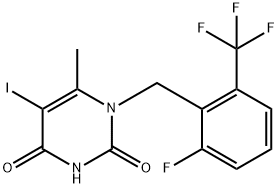
1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione synthesis
- Product Name:1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione
- CAS Number:1150560-54-5
- Molecular formula:C13H9F4IN2O2
- Molecular Weight:428.12
1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical generation process. Its synthesis method is that :To 1-(2-fluoro-6- trifluoromethyl-benzyl)-6-methyl-lH-pyrimidine-2,4-dione inmethanol, Iodine monochloride was added and heated at 50 ℃ for 3 hours. The mixture was filtered and washed with methanol. The off-white solid was dried to provide 1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione (90% yield).
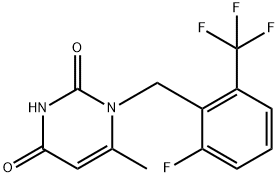
830346-47-9
194 suppliers
$24.00/100mg
![1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione](/CAS/GIF/1150560-54-5.gif)
1150560-54-5
158 suppliers
inquiry
Yield:1150560-54-5 90.5%
Reaction Conditions:
with N-iodo-succinimide;acetic acid at 50; for 8 h;
Steps:
1 Example 1: Preparation of the Compound of Formula III
To a suitable reactor, the compound of formula II (90.0 g) and acetic acid (900 mL) were added at room temperature. The resulting clear solution was added N-iodosuccinimide (80.4 g). The mixture was heated to 50° C. and stirred for 8 hours. Upon completion, the suspension was added water (2250 mL) slowly and then cooled to room temperature. The slurry was stirred at room temperature for 2 hours, followed by filtration. The wet cake was washed with water (540 mL) twice and then dried in vacuo at 60° C. to afford crude compound of formula III. Crude compound of formula III and MeOH (225 mL) was charged to a suitable reactor. The slurry mixture was heated to reflux and stirred for 1 hour. After reflux, the slurry was cooled to room temperature and stirred for 1 hour, followed by filtration. The wet cake was washed with pre-cooled MeOH (90 mL) and then dried in vacuo at 60° C. to afford the compound of formula III (115.38 g, 90.5% yield).
References:
ScinoPharm Taiwan, Ltd. US2021/78956, 2021, A1 Location in patent:Paragraph 0117; 0118
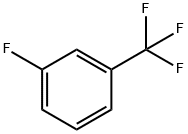
401-80-9
313 suppliers
$5.00/10g
![1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione](/CAS/GIF/1150560-54-5.gif)
1150560-54-5
158 suppliers
inquiry
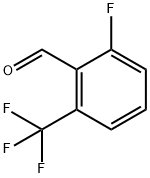
60611-24-7
202 suppliers
$10.00/1g
![1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione](/CAS/GIF/1150560-54-5.gif)
1150560-54-5
158 suppliers
inquiry
581072-15-3
6 suppliers
inquiry
![1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione](/CAS/GIF/1150560-54-5.gif)
1150560-54-5
158 suppliers
inquiry
239087-06-0
178 suppliers
$14.00/100mg
![1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione](/CAS/GIF/1150560-54-5.gif)
1150560-54-5
158 suppliers
inquiry