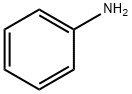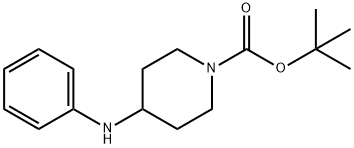
1-N-Boc-4-(Phenylamino)piperidine synthesis
- Product Name:1-N-Boc-4-(Phenylamino)piperidine
- CAS Number:125541-22-2
- Molecular formula:C16H24N2O2
- Molecular Weight:276.37
79099-07-3
0 suppliers
$5.00/5g

125541-22-2
0 suppliers
$31.00/1g
Yield:125541-22-2 98%
Reaction Conditions:
with sodium hydroxide;sodium tris(acetoxy)borohydride;glacial acetic acid;aniline in diethyl ether;dichloromethane;
Steps:
4-Phenylamino-piperidine-1-carboxylic Acid Tert.-butyl Ester
Reaction Scheme 1, Step 1 4-Phenylamino-piperidine-1-carboxylic Acid Tert.-butyl Ester A solution of N-tert-butoxycarbonyl-4-piperidone (Lancaster 13361, 7 g) and aniline (Aldrich 24228-4, 3.3 g) in dichloromethane (200 ml) was treated with sodium triacetoxyborohydride (Aldrich 31639, 10.4 g) followed by acetic acid (2.1 g) and the mixture stirred for 2 h at ambient temperature. 1M Aqueous sodium hydroxide solution (100 ml) was added, followed by diethyl ether (200 ml) and the mixture stirred vigorously for 5 min. The organic phase was separated, washed with water (100 ml), followed by brine (100 ml), dried (anhydrous magnesium sulphate), filtered and evaporated to give the title compound as a white solid (9.5 g, 98%). Mass spectrum 277 [M+H]+.
References:
US2003/69276,2003,A1
1228630-89-4
54 suppliers
$55.00/50mg
98-80-6
747 suppliers
$5.00/5g

125541-22-2
0 suppliers
$31.00/1g
85908-96-9
195 suppliers
$6.00/1g

125541-22-2
0 suppliers
$31.00/1g
150008-24-5
70 suppliers
$11.00/1g

125541-22-2
0 suppliers
$31.00/1g