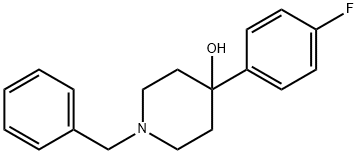
1-BENZYL-4-(4-FLUOROPHENYL)PIPERIDIN-4-OL synthesis
- Product Name:1-BENZYL-4-(4-FLUOROPHENYL)PIPERIDIN-4-OL
- CAS Number:163631-02-5
- Molecular formula:C18H20FNO
- Molecular Weight:285.36
Yield:163631-02-5 98%
Reaction Conditions:
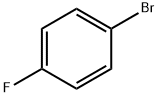
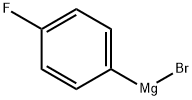
Stage #1: 1-Bromo-4-fluorobenzenewith iodine;magnesium in tetrahydrofuran;Inert atmosphere;Reflux;Grignard reaction;
Stage #2: 1-phenylmethyl-4-piperidone in tetrahydrofuran at 20; for 3 h;Inert atmosphere;Cooling with ice;Grignard reaction;
Steps:
Preparation of 1-benzyl-4-arylpiperidin-4-ols (General Procedure A)
General procedure: All glassware used in the following procedure was flame dried and then cooled under nitrogen prior to use. A portion (1 mmol) of the required aryl bromide (10.00 mmol) was added to a suspension of magnesium turnings (250 mg, 10.5 mmol) in tetrahydrofuran (12 mL) containing a few iodine crystals and the mixture gently heated whilst stirring vigorously. Once the reaction forming the Grignard reagent had initiated (reflux became self-sustaining) the remaining bromobenzene was added dropwise at such a rate that gentle reflux was maintained. The mixture was then heated at reflux until the magnesium was consumed (usually 30-60 min) and then was cooled for 30 min using an ice bath. A solution of 1-benzyl 4-piperidone (5.00 - 6.25 mmol) in tetrahydrofuran (8 mL) was added dropwise over 1-2 h and the mixture was stirred for 1 h and then the ice bath was removed and the reaction mixture allowed to warm to room temperature whilst stirring for at least a further 2 h. The reaction was quenched by the addition of saturated ammonium chloride solution (60 mL) and extracted into diethyl ether (2 × 30 mL) followed by ethyl acetate (3 × 30 mL). The organic phases were combined and subjected to standard work up conditions to give the required crude product. Purification of the title compound was achieved by flash chromatography on silica or, where indicated, by elution through a short length silica column using the eluant specified. In some cases, the crude piperidinol was dehydrated directly without purification as indicated.
References:
Conway, Richard J.;Valant, Celine;Christopoulos, Arthur;Robertson, Alan D.;Capuano, Ben;Crosby, Ian T. [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 7,p. 2560 - 2564] Location in patent:supporting information; experimental part
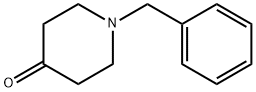
3612-20-2
488 suppliers
$6.00/10g
352-13-6
197 suppliers
$40.00/50mL

163631-02-5
13 suppliers
inquiry
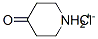
41979-39-9
116 suppliers
$15.00/10mg

163631-02-5
13 suppliers
inquiry