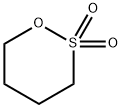
1,4-Butane sultone synthesis
- Product Name:1,4-Butane sultone
- CAS Number:1633-83-6
- Molecular formula:C4H8O3S
- Molecular Weight:136.17
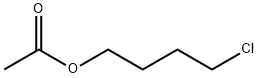
6962-92-1
211 suppliers
$6.00/5g

1633-83-6
416 suppliers
$17.00/5g
Yield:1633-83-6 92%
Reaction Conditions:
Stage #1: 4-Chlorobutyl acetatewith sodium sulfite in water;Reflux;
Stage #2: with hydrogenchloride in methanol; for 4 h;Reflux;
Stage #3: at 110; under 20 Torr;Temperature;Pressure;Solvent;
Steps:
1-4 Example 2: Preparation of 1,4-butanesultone
A mixture of 4-chlorobutyl acetate (20g), sodium sulphite (25.5g) and water (86g) were refluxed for 4-6 hours. Water was removed using vacuum and subsequently methanol (64g) was added to the reaction mass. Anhydrous hydrochloric acid was passed for 4 hours at reflux temperature and stirred. The reaction mass was filtered and filtrate was concentrated using rotary evaporator to remove methanol to obtain crude mass. The crude mass was dehydrated under vacuum (20 torr) at 110°C in absence of solvent until complete conversion to 1,4-butane sultone takes place. Reaction mass was cooled to room temperature and dichloromethane and water were added. Organic and aqueous layers were separated and aqueous layer was washed with dichloromethane and separated. The combined organic layer was concentrated using rotary evaporator to give pure 1,4-butane sultone.Yield: 92 %Purity: 99.8%
References:
WO2021/33200,2021,A1 Location in patent:Page/Page column 7-9
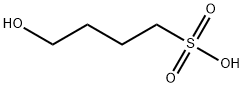
26978-64-3
115 suppliers
$245.00/5g

1633-83-6
416 suppliers
$17.00/5g

928-51-8
339 suppliers
$6.00/5g

1633-83-6
416 suppliers
$17.00/5g
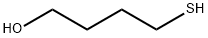
14970-83-3
58 suppliers
inquiry

1633-83-6
416 suppliers
$17.00/5g
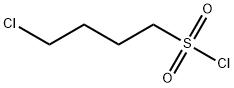
1633-84-7
68 suppliers
$23.00/100mg
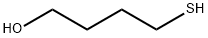
14970-83-3
58 suppliers
inquiry

1633-83-6
416 suppliers
$17.00/5g

1633-84-7
68 suppliers
$23.00/100mg
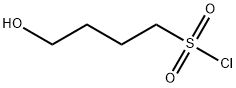
113309-30-1
3 suppliers
inquiry