ChemicalBook >?? ???? >???? ??
???? ??
|
|
???? ?? ??
- ???
- decomposes at 270℃ [HAW93]
- ??
- 2.650
- ???
- ???, ???? ???
- ??? ??
- ?? ??
- ??
- ???
- ???
- ?? ?????.
- ??
- Hygroscopic
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | O,Xi | ||
|---|---|---|---|
| ?? ???? ?? | 36/37/38 | ||
| ????? | 26-36 | ||
| ????(UN No.) | UN 1455 5.1/PG 2 | ||
| WGK ?? | 3 | ||
| TSCA | Yes | ||
| ?? ?? | 5.1 | ||
| ???? | II | ||
| ???? ?? | KE-04595 |
???? ?? C??? ??, ??, ??
??
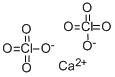
The anhydrate is a white to yellow crystalline solid with a density of 2.651 g/cm3. Contact with this salt will irritate the eyes, skin and mucus membranes. If ingested, it is toxic in sufficient quantities. When formed from an aqueous solution, the tetrahydrate is obtained. Calcium perchlorate tetrahydrate has the formula Ca(ClO4)2·4H2O and the CAS number 15627-86-8. Its molecular weight is 311.04 g/mol and its density is 2.651 g/cm3. Its solubility in water is 188 g/ 100 ml at 20°C.??? ??
white crystal(s); oxidizing agent [HAW93]??
Calcium perchlorate hydrate is used as a pharmaceutical intermediate.?? ??
Calcium perchlorate can be prepared by reacting calcium carbonate with a solution of perchloric acid:CaCO3+ HClO4→Ca(ClO4)2+ CO2
This salt can also be prepared by an electrochemical method in water whereby calcium perchlorate is formed from calcium chlorate in which a platinum anode and a rotating stainless steel cathode is employed.
?? ??
White to yellow crystalline solid. Density 2.651 g / cm3. Contact may irritate skin, eyes, and mucous membranes. May be toxic by ingestion.??? ?? ??
Water soluble.?? ???
A strong oxidizing agent. Can react with reducing agents to generate heat and products that may be gaseous (causing pressurization in closed containers). The products may themselves be capable of further reactions (such as combustion in the air). Redox reactions can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Consequently explosive mixtures with reducing agents often persist unchanged for long periods. Such systems are typically mixtures of solids, but may involve any combination of physical states. Can react violently with active metals, cyanides, esters, and thiocyanates. May cause the acceleration of the burning of combustible materials. Prolonged exposure to heat of flames may result in an explosion.????
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.????
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.???? ?? ?? ?? ? ???
???
?? ??
???? ?? ?? ??
???( 34)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 8670 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 |
bess@weibangbio.com | China | 18147 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 |
sales@aladdinsci.com | United States | 57505 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32343 | 55 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 |
chemwill_asia@126.com | CHINA | 23912 | 58 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 021-61259108 18621169109 |
market03@meryer.com | China | 40228 | 62 |
| Alfa Aesar | 400-6106006 |
saleschina@alfa-asia.com | China | 30123 | 84 |
| Energy Chemical | 021-021-58432009 400-005-6266 |
sales8178@energy-chemical.com | China | 44689 | 61 |
| Shanghai Aladdin Bio-Chem Technology Co.,LTD | 400-6206333 18021050169 |
market@aladdin-e.com | China | 25003 | 65 |
???? ?? ?? ??:
????? ?? ??? ?????? ???? ???? ??? ???? ????? ???? ???? ????? ????? ???? ???? ??
MAGNESIUM PERCHLORATE HEXAHYDRATE
CALCIUM PERCHLORATE,CALCIUM PERCHLORATE, HYDRATED,Calcium perchlorate tetrahydrate, pure
MAGNESIUM PERCHLORATE DIHYDRATE
Dihypochlorous acid strontium salt
RADIUM CHLORATE
beryllium perchlorate
Calcium chlorate dihydrate
CALCIUM PERCHLORATE HYDRATE