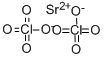ChemicalBook >?? ???? >????????
????????
|
|
???????? ??
- ???
- <100°C
- ???
- ???, ???? ???
- ??? ??
- ?? ??
- ??
- ???
- ???
- mol/kg H2O: 8.16(0°C), 10.67(25°C), 12.70(40°C); ???, Sr(ClO4)2 · 4H2O (0°C), Sr(ClO4)2 ·2H2O (25°C), 3Sr(ClO4)2 · 2H2O (40°C ??) [KRU93]; ??? ??? [HAW93]
- ???
- ???
- CAS ??????
- 13450-97-0(CAS DataBase Reference)
??
| ??? ?? | Xi | ||
|---|---|---|---|
| ?? ???? ?? | 8-34-36/38 | ||
| ????? | 17-26-36/37/39 | ||
| ????(UN No.) | 1508 | ||
| ?? ?? | 5.1 | ||
| ???? | II | ||
| ???? ?? | KE-32240 |
???????? C??? ??, ??, ??
??? ??
white crystal(s); hygroscopic [STR93]??? ??
Strontium perchlorate has the formula, Sr(ClO4)2 and a molecular weight of 286.52 g/mol. It is a white solid with a melting point of 100°C. Its density is 2.073 g/ cm3 and it is lightly soluble in water (3.21 g/100 ml at 20°C). It can be prepared by reaction of strontium carbonate with a solution of perchloric acid or ammonium perchlorate:SrCO3+ 2HClO4→Sr(ClO4)2+H2O+ CO2
In an aqueous solution, the hexahydrate results.
Sr(ClO4)2·6H2O has the molecular weight of 394.63 g/ mol and a CAS number of 13450-97-0. A trihydrate, Sr(ClO4)2·3H2O is also known, having the molecular weight of 340.57 g/mol and a CAS number of 15650- 09-6.
?? ??
A colorless crystalline powder. Dangerous fire risk when in contact with organic materials. Used to make other chemicals.??? ?? ??
Denser than water and soluble in water.?? ???
Oxidizing agents, such as STRONTIUM PERCHLORATE, can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds, in general, have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates.????
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.????
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.???????? ?? ?? ? ???
???
?? ??
???????? ?? ??
???( 35)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Aladdin Scientific | |
tp@aladdinsci.com | United States | 52924 | 58 |
| XIAMEN AMITY INDUSTRY AND TRADE CO., LTD. | +8618950047208 |
ellena@amitychem.com | China | 43416 | 58 |
| DAYANG CHEM (HANGZHOU) CO.,LTD | +86-88938639 +86-17705817739 |
info@dycnchem.com | China | 53900 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32343 | 55 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 021-61259108 18621169109 |
market03@meryer.com | China | 40228 | 62 |
| 821-50328103-801 18930552037 |
3bsc@sina.com | China | 15839 | 69 | |
| Shanghai Na Qian Chemical Technology Co. Ltd. | 0373-7129549; 13598610367 |
2841375912@qq.com | China | 2731 | 52 |
| Guangdong Wengjiang Chemical Reagent Co., Ltd. | 0751-2886766 13927870850 |
3001267247@qq.com | China | 9947 | 58 |
???????? ?? ??:
????? ?? ??? ?????? ???? ???? ?? ?????? ????? ? ????? ???? ?? ???????? ????
MAGNESIUM PERCHLORATE DIHYDRATE
CALCIUM PERCHLORATE
MAGNESIUM PERCHLORATE HEXAHYDRATE
RADIUM CHLORATE
beryllium perchlorate
Dihypochlorous acid strontium salt
STRONTIUM PERCHLORATE, TRIHYDRATE,STRONTIUM PERCHLORATE, TRIHYDRATE REAGENT
STRONTIUM PERCHLORATE HEXAHYDRATE
STRONTIUM PERCHLORATE HYDRATE