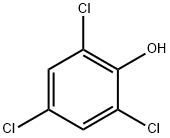
2,4,6-???????
|
|
2,4,6-??????? ??
- ???
- 64-66 °C(lit.)
- ?? ?
- 246 °C(lit.)
- ??
- 1.49
- ???
- 1 mm Hg ( 76.5 °C)
- ???
- 1.5300 (estimate)
- ???
- 99 °C
- ?? ??
- Store below +30°C.
- ???
- 0.8g/L
- ??? ??
- ??? ??? ?? ??? ?? ? ???
- ?? ?? (pKa)
- 6.15 (Leuenberger et al., 1985)
6.10 (Blackman et al., 1955)
6.0 (Eder and Weber, 1980)
- ??
- ???? ?? ??
- ???
- 0.8g/L
- Merck
- 14,9644
- BRN
- 776729
- Henry's Law Constant
- 9.07 at 25 °C (estimated, Leuenberger et al., 1985a)
- CAS ??????
- 88-06-2(CAS DataBase Reference)
- IARC
- 2B (Vol. 117) 2019
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xn,N,T,F | ||
|---|---|---|---|
| ?? ???? ?? | 22-36/38-50/53-40-52/53-39/23/24/25-23/24/25-11-51/53 | ||
| ????? | 36/37-60-61-45-16 | ||
| ????(UN No.) | UN 3077 9/PG 3 | ||
| WGK ?? | 3 | ||
| RTECS ?? | SN1575000 | ||
| TSCA | Yes | ||
| ?? ?? | 6.1 | ||
| ???? | III | ||
| HS ?? | 29081000 | ||
| ?? ?? ??? | 88-06-2(Hazardous Substances Data) | ||
| ?? | LD50 orally in Rabbit: 887 mg/kg | ||
| 発がん性評(píng)価について | EPA B2 | ||
| ???? ?? | KE-34087 |
2,4,6-??????? C??? ??, ??, ??
??? ??
Yellow flakes; strong phenolic odor. Soluble in acetone, alcohol, and ether; insoluble in water. Nonflammable.??? ??
Colorless needles or yellow solid with a strong, phenolic, musty or rotten vegetable-type odor. At 40 °C, the lowest concentration at which an odor was detected was 380 μg/L. At 25 °C, the lowest concentration at which a taste was detected was >12 μg/L (Young et al., 1996).??
2,4,6-Trichlorophenol is used as a broad range pesticide against insects, fungi, vegetation and bacteria. It has become a common environmental contaminant and probable human carcinogen.??
ChEBI: A trichlorophenol with phenolic substituents on positions 2, 4 and 6.??? ?? ??
Insoluble in water.?? ???
2,4,6-Trichlorophenol is incompatible with acid chlorides, acid anhydrides and oxidizing agents. 2,4,6-Trichlorophenol can be converted to the sodium salt by reaction with sodium carbonate. Forms ethers, esters and salts by reaction with metals and amines. Undergoes substitution reactions such as nitration, alkylation, acetylation and halogenation. Can be hydrolyzed by reaction with bases at elevated temperatures and pressures. Reacts with alkalis at high temperatures .????
In experimental animals, 2,4,6- trichlorophenol causes toxic effects to the liver and hematologic system and cancer. There is no reliable information regarding exposure and toxic effects in humans.????
Literature sources indicate that 2,4,6-Trichlorophenol is nonflammable.Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Poison by intraperitoneal route. Moderately toxic by ingestion and skin contact. A skin and severe eye irritant. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of Cl-. Used as a germicide and preservative. See also CHLOROPHENOLS.Carcinogenicity
2,4,6-Trichlorophenol is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.????
Biological. In activated sludge, only 0.3% mineralized to carbon dioxide after 5 d (Freitag et al., 1985). In anaerobic sludge, 2,4,6-trichlorophenol degraded to 4-chlorophenol (Mikesell and Boyd, 1985). When 2,4,6-trichlorophenol was statically incubated in the dark at 25 °C with yeast extract and settled domestic wastewater inoculum, significant biodegradation with rapid adaptation was observed. At concentrations of 5 and 10 mg/L, 96 and 97% biodegradation, respectively, were observed after 7 d (Tabak et al., 1981).Photolytic. Titanium dioxide suspended in an aqueous solution and irradiated with UV light (λ = 365 nm) converted 2,4,6-trinitrophenol to carbon dioxide at a significant rate (Matthews, 1986). A carbon dioxide yield of 65.8% was achieved when 2,4,6-trichlorophenol adsorbed on silica gel was irradiated with light (λ >290 nm) for 17 h (Freitag et al., 1985).
Chemical/Physical. An aqueous solution containing chloramine reacted with 2,4,6-trichlorophenol to yield the following intermediate products after 2 h at 25 °C: 2,6-dichloro-1,4- benzoquinone-4-(N-chloro)imine and 4,6-dichloro-1,2-benzoquinone-2-(N-chloro)imine (Maeda et al., 1987).
Purification Methods
Crystallise the phenol from *benzene, EtOH or EtOH/water. [Beilstein 6 IV 1005.]2,4,6-??????? ?? ?? ? ???
???
?? ??
2,4,6-??????? ?? ??
???( 335)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 |
sales@myuchem.com | China | 7696 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5876 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29730 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29863 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 |
sales@alchempharmtech.com | United States | 63687 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49732 | 58 |
| Fuxin Pharmaceutical | +86-021-021-50872116 +8613122107989 |
contact@fuxinpharm.com | China | 10035 | 58 |